Bohr–Sommerfeld Model on:
[Wikipedia]
[Google]
[Amazon]
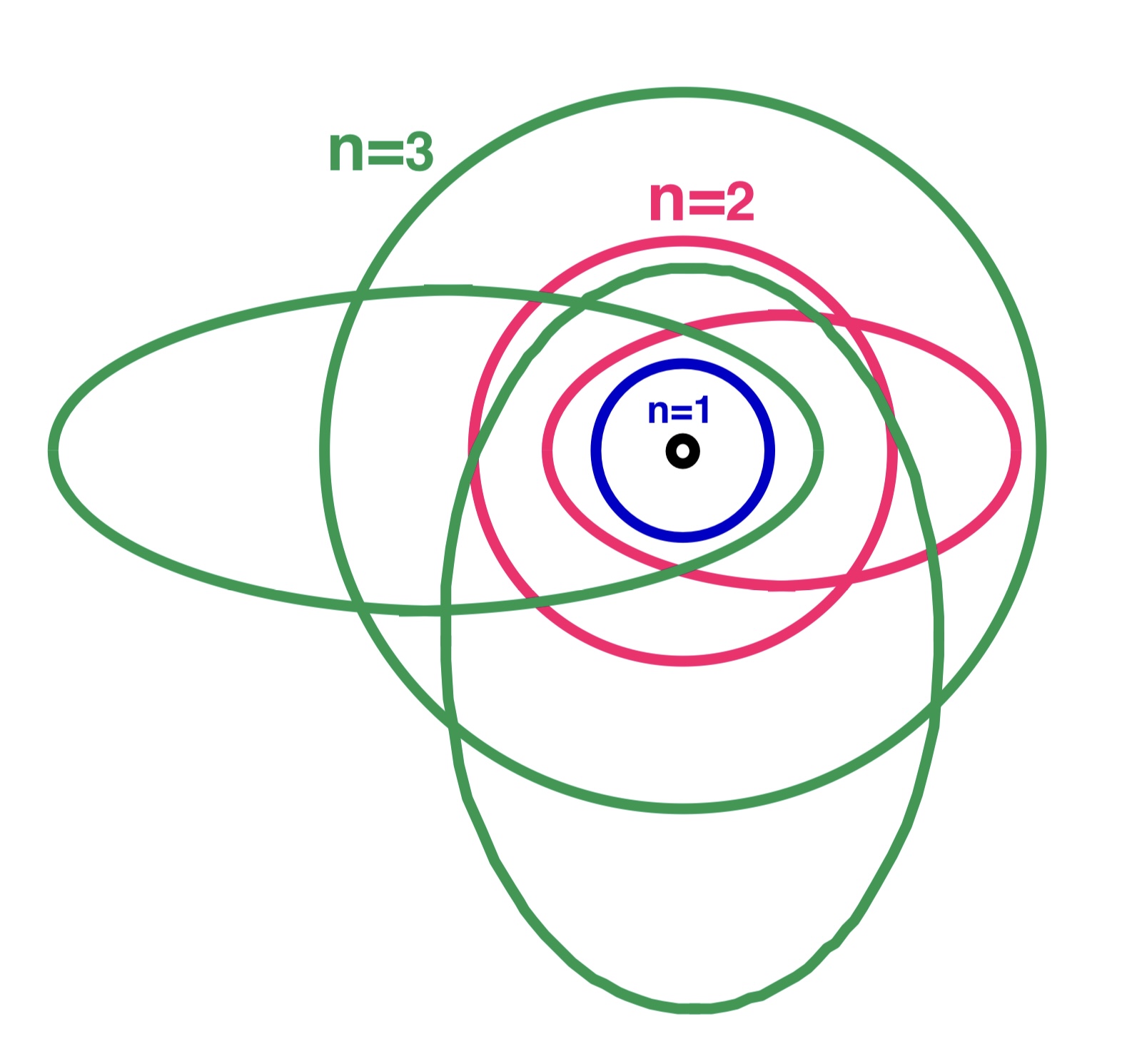 The Bohr–Sommerfeld model (also known as the Sommerfeld model or Bohr–Sommerfeld theory) was an extension of the
The Bohr–Sommerfeld model (also known as the Sommerfeld model or Bohr–Sommerfeld theory) was an extension of the

 The Bohr–Sommerfeld model (also known as the Sommerfeld model or Bohr–Sommerfeld theory) was an extension of the
The Bohr–Sommerfeld model (also known as the Sommerfeld model or Bohr–Sommerfeld theory) was an extension of the Bohr model
In atomic physics, the Bohr model or Rutherford–Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar Sy ...
to allow elliptical orbits of electrons around an atomic nucleus. Bohr–Sommerfeld theory is named after Danish physicist Niels Bohr
Niels Henrik David Bohr (; 7 October 1885 – 18 November 1962) was a Danish physicist who made foundational contributions to understanding atomic structure and quantum theory, for which he received the Nobel Prize in Physics in 1922 ...
and German physicist Arnold Sommerfeld
Arnold Johannes Wilhelm Sommerfeld, (; 5 December 1868 – 26 April 1951) was a German theoretical physicist who pioneered developments in atomic and quantum physics, and also educated and mentored many students for the new era of theoretic ...
. Sommerfeld argued that if electronic orbits could be elliptical instead of circular, the energy of the electron would be the same, except in the presence of a magnetic field, introducing what is now known as quantum degeneracy
In quantum mechanics, an energy level is degenerate if it corresponds to two or more different measurable states of a quantum system. Conversely, two or more different states of a quantum mechanical system are said to be degenerate if they give th ...
.
The Bohr–Sommerfeld model supplemented the quantized angular momentum condition of the Bohr model with an additional radial quantization condition, the Wilson
Wilson may refer to:
People
*Wilson (name)
** List of people with given name Wilson
** List of people with surname Wilson
* Wilson (footballer, 1927–1998), Brazilian manager and defender
*Wilson (footballer, born 1984), full name Wilson Rod ...
– Sommerfeld quantization condition
:
where ''pr'' is the radial momentum canonically conjugate to the coordinate ''q'', which is the radial position, and ''T'' is one full orbital period. The integral is the action
Action may refer to:
* Action (narrative), a literary mode
* Action fiction, a type of genre fiction
* Action game, a genre of video game
Film
* Action film, a genre of film
* ''Action'' (1921 film), a film by John Ford
* ''Action'' (1980 fil ...
of action-angle coordinates
In classical mechanics, action-angle coordinates are a set of canonical coordinates useful in solving many integrable systems. The method of action-angles is useful for obtaining the frequencies of oscillatory or rotational motion without solving ...
. This condition, suggested by the correspondence principle
In physics, the correspondence principle states that the behavior of systems described by the theory of quantum mechanics (or by the old quantum theory) reproduces classical physics in the limit of large quantum numbers. In other words, it says ...
, is the only one possible, since the quantum numbers are adiabatic invariant
A property of a physical system, such as the entropy of a gas, that stays approximately constant when changes occur slowly is called an adiabatic invariant. By this it is meant that if a system is varied between two end points, as the time for the ...
s.
History
In 1913,Niels Bohr
Niels Henrik David Bohr (; 7 October 1885 – 18 November 1962) was a Danish physicist who made foundational contributions to understanding atomic structure and quantum theory, for which he received the Nobel Prize in Physics in 1922 ...
displayed rudiments of the later defined correspondence principle
In physics, the correspondence principle states that the behavior of systems described by the theory of quantum mechanics (or by the old quantum theory) reproduces classical physics in the limit of large quantum numbers. In other words, it says ...
and used it to formulate a model
A model is an informative representation of an object, person or system. The term originally denoted the plans of a building in late 16th-century English, and derived via French and Italian ultimately from Latin ''modulus'', a measure.
Models c ...
of the hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen cons ...
which explained the line spectrum
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a transition from a high energy state to a lower energy state. The photon energy ...
. In the next few years Arnold Sommerfeld
Arnold Johannes Wilhelm Sommerfeld, (; 5 December 1868 – 26 April 1951) was a German theoretical physicist who pioneered developments in atomic and quantum physics, and also educated and mentored many students for the new era of theoretic ...
extended the quantum rule to arbitrary integrable systems making use of the principle of adiabatic invariance of the quantum numbers introduced by Lorentz and Einstein. Sommerfeld made a crucial contribution by quantizing the z-component of the angular momentum
In physics, angular momentum (rarely, moment of momentum or rotational momentum) is the rotational analog of linear momentum. It is an important physical quantity because it is a conserved quantity—the total angular momentum of a closed sy ...
, which in the old quantum era was called "space quantization" (German: ''Richtungsquantelung''). This allowed the orbits of the electron to be ellipses instead of circles, and introduced the concept of quantum degeneracy. The theory would have correctly explained the Zeeman effect
The Zeeman effect (; ) is the effect of splitting of a spectral line into several components in the presence of a static magnetic field. It is named after the Dutch physicist Pieter Zeeman, who discovered it in 1896 and received a Nobel priz ...
, except for the issue of electron spin. Sommerfeld's model was much closer to the modern quantum mechanical picture than Bohr's.
In the 1950s Joseph Keller
Joseph Bishop Keller (July 31, 1923 – September 7, 2016) was an American mathematician who specialized in applied mathematics. He was best known for his work on the "geometrical theory of diffraction" (GTD).
Early life and education
Born i ...
updated Bohr–Sommerfeld quantization using Einstein's interpretation of 1917, now known as Einstein–Brillouin–Keller method
The Einstein–Brillouin–Keller method (EBK) is a semiclassical method (named after Albert Einstein, Léon Brillouin, and Joseph B. Keller) used to compute eigenvalues in quantum-mechanical systems. EBK quantization is an improvement from B ...
. In 1971, Martin Gutzwiller Martin may refer to:
Places
* Martin City (disambiguation)
* Martin County (disambiguation)
* Martin Township (disambiguation)
Antarctica
* Martin Peninsula, Marie Byrd Land
* Port Martin, Adelie Land
* Point Martin, South Orkney Islands
Austr ...
took into account that this method only works for integrable systems and derived a semiclassical way of quantizing chaotic systems from path integrals.
Predictions
The Sommerfeld model predicted that the magnetic moment of an atom measured along an axis will only take on discrete values, a result which seems to contradict rotational invariance but which was confirmed by theStern–Gerlach experiment
The Stern–Gerlach experiment demonstrated that the spatial orientation of angular momentum is quantized. Thus an atomic-scale system was shown to have intrinsically quantum properties. In the original experiment, silver atoms were sent throug ...
. This was a significant step in the development of quantum mechanics. It also described the possibility of atomic energy levels
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The t ...
being split by a magnetic field
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and t ...
(called the Zeeman effect). Walther Kossel
Walther Ludwig Julius Kossel (4 January 1888 – 22 May 1956) was a German physicist known for his theory of the chemical bond ( ionic bond/octet rule), Sommerfeld–Kossel displacement law of atomic spectra, the Kossel-Stranski model for crystal ...
worked with Bohr and Sommerfeld on the Bohr–Sommerfeld model of the atom introducing two electrons in the first shell and eight in the second.
Issues
The Bohr–Sommerfeld model was fundamentally inconsistent and led to many paradoxes. Themagnetic quantum number
In atomic physics, the magnetic quantum number () is one of the four quantum numbers (the other three being the principal, azimuthal, and spin) which describe the unique quantum state of an electron. The magnetic quantum number distinguishes ...
measured the tilt of the orbital plane relative to the ''xy'' plane, and it could only take a few discrete values. This contradicted the obvious fact that an atom could be turned this way and that relative to the coordinates without restriction. The Sommerfeld quantization can be performed in different canonical coordinates and sometimes gives different answers. The incorporation of radiation corrections was difficult, because it required finding action-angle coordinates for a combined radiation/atom system, which is difficult when the radiation is allowed to escape. The whole theory did not extend to non-integrable motions, which meant that many systems could not be treated even in principle. In the end, the model was replaced by the modern quantum-mechanical treatment of the hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen cons ...
, which was first given by Wolfgang Pauli
Wolfgang Ernst Pauli (; ; 25 April 1900 – 15 December 1958) was an Austrian theoretical physicist and one of the pioneers of quantum physics. In 1945, after having been nominated by Albert Einstein, Pauli received the Nobel Prize in Physics fo ...
in 1925, using Heisenberg's matrix mechanics
Matrix mechanics is a formulation of quantum mechanics created by Werner Heisenberg, Max Born, and Pascual Jordan in 1925. It was the first conceptually autonomous and logically consistent formulation of quantum mechanics. Its account of quantum ...
. The current picture of the hydrogen atom is based on the atomic orbitals
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any sp ...
of wave mechanics Wave mechanics may refer to:
* the mechanics of waves
* the ''wave equation'' in quantum physics, see Schrödinger equation
See also
* Quantum mechanics
* Wave equation
The (two-way) wave equation is a second-order linear partial differenti ...
, which Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger (, ; ; 12 August 1887 – 4 January 1961), sometimes written as or , was a Nobel Prize-winning Austrian physicist with Irish citizenship who developed a number of fundamental results in quantum theo ...
developed in 1926.
However, this is not to say that the Bohr–Sommerfeld model was without its successes. Calculations based on the Bohr–Sommerfeld model were able to accurately explain a number of more complex atomic spectral effects. For example, up to first-order perturbations
Perturbation or perturb may refer to:
* Perturbation theory, mathematical methods that give approximate solutions to problems that cannot be solved exactly
* Perturbation (geology), changes in the nature of alluvial deposits over time
* Perturbatio ...
, the Bohr model and quantum mechanics make the same predictions for the spectral line splitting in the Stark effect
The Stark effect is the shifting and splitting of spectral lines of atoms and molecules due to the presence of an external electric field. It is the electric-field analogue of the Zeeman effect, where a spectral line is split into several compo ...
. At higher-order perturbations, however, the Bohr model and quantum mechanics differ, and measurements of the Stark effect under high field strengths helped confirm the correctness of quantum mechanics over the Bohr model. The prevailing theory behind this difference lies in the shapes of the orbitals of the electrons, which vary according to the energy state of the electron.
The Bohr–Sommerfeld quantization conditions lead to questions in modern mathematics. Consistent semiclassical quantization condition requires a certain type of structure on the phase space, which places topological limitations on the types of symplectic manifolds which can be quantized. In particular, the symplectic form should be the curvature form In differential geometry, the curvature form describes curvature of a connection on a principal bundle. The Riemann curvature tensor in Riemannian geometry can be considered as a special case.
Definition
Let ''G'' be a Lie group with Lie alg ...
of a connection of a Hermitian {{Short description, none
Numerous things are named after the French mathematician Charles Hermite (1822–1901):
Hermite
* Cubic Hermite spline, a type of third-degree spline
* Gauss–Hermite quadrature, an extension of Gaussian quadrature m ...
line bundle
In mathematics, a line bundle expresses the concept of a line that varies from point to point of a space. For example, a curve in the plane having a tangent line at each point determines a varying line: the ''tangent bundle'' is a way of organisin ...
, which is called a prequantization.
Relativistic orbit
Arnold Sommerfeld
Arnold Johannes Wilhelm Sommerfeld, (; 5 December 1868 – 26 April 1951) was a German theoretical physicist who pioneered developments in atomic and quantum physics, and also educated and mentored many students for the new era of theoretic ...
derived the relativistic solution of atomic energy levels. We will start this derivationhttps://archive.org/details/atombauundspekt00sommgoog/page/n541 - Atombau und Spektrallinien, 1921, page 520 with the relativistic equation for energy in the electric potential
The electric potential (also called the ''electric field potential'', potential drop, the electrostatic potential) is defined as the amount of work energy needed to move a unit of electric charge from a reference point to the specific point in ...
:
After substitution we get
:
For momentum , and their ratio the equation of motion is (see Binet equation)
:
with solution
:
The angular shift of periapsis
An apsis (; ) is the farthest or nearest point in the orbit of a planetary body about its primary body. For example, the apsides of the Earth are called the aphelion and perihelion.
General description
There are two apsides in any ellip ...
per revolution is given by
:
With the quantum conditions
:
and
:
we will obtain energies
:
where is the fine-structure constant
In physics, the fine-structure constant, also known as the Sommerfeld constant, commonly denoted by (the Greek letter ''alpha''), is a fundamental physical constant which quantifies the strength of the electromagnetic interaction between ele ...
. This solution (using substitutions for quantum numbers) is equivalent to the solution of the Dirac equation
In particle physics, the Dirac equation is a relativistic wave equation derived by British physicist Paul Dirac in 1928. In its free form, or including electromagnetic interactions, it describes all spin- massive particles, called "Dirac pa ...
. Nevertheless, both solutions fail to predict the Lamb shift
In physics, the Lamb shift, named after Willis Lamb, is a difference in energy between two energy levels 2''S''1/2 and 2''P''1/2 (in term symbol notation) of the hydrogen atom which was not predicted by the Dirac equation, according to which ...
s.
See also
*Bohr model
In atomic physics, the Bohr model or Rutherford–Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar Sy ...
* Old quantum theory
The old quantum theory is a collection of results from the years 1900–1925 which predate modern quantum mechanics. The theory was never complete or self-consistent, but was rather a set of heuristic corrections to classical mechanics. The theory ...
References