Birch Alkylation on:
[Wikipedia]
[Google]
[Amazon]
The Birch reduction is an organic reaction that is used to convert arenes to cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch and involves the organic reduction of aromatic rings in an amine solvent (traditionally liquid ammonia) with an  An example is the reduction of
An example is the reduction of 

 Solvated electrons will preferentially reduce sufficiently electronegative functional groups, such as
Solvated electrons will preferentially reduce sufficiently electronegative functional groups, such as
 Simple Hückel computations lead to equal electron densities at the three atoms 1, 3 and 5, but asymmetric bond orders. Modifying the exchange integrals to account for varying interatomic distances, produces maximum electron density at the central atom 1, a result confirmed by more modern RHF computations.
*
*
The result is analogous to conjugated enolates. When those anions (but not the enol
Simple Hückel computations lead to equal electron densities at the three atoms 1, 3 and 5, but asymmetric bond orders. Modifying the exchange integrals to account for varying interatomic distances, produces maximum electron density at the central atom 1, a result confirmed by more modern RHF computations.
*
*
The result is analogous to conjugated enolates. When those anions (but not the enol
 In substituted aromatics, an
In substituted aromatics, an 
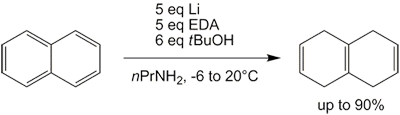 The directing effects of naphthalene substituents remain relatively unstudied theoretically. Substituents adjacent to the bridge appear to direct reduction to the unsubstituted ring; β substituents (one bond further) tend to direct reduction to the substituted ring.
The directing effects of naphthalene substituents remain relatively unstudied theoretically. Substituents adjacent to the bridge appear to direct reduction to the unsubstituted ring; β substituents (one bond further) tend to direct reduction to the substituted ring.
 For electron-donating substituents, Birch initially proposed ''meta'' attack, corresponding to the location of greatest electron density in a neutral benzene ring, a position endorsed by Krapcho and Bothner-By. These conclusions were challenged by Zimmerman in 1961, who computed electron densities of the radical and diene anions, revealing that the ''ortho'' site which was most negative and thus most likely to protonate. But the situation remained uncertain, because computations remained highly sensitive to transition geometry. Worse, Hückel orbital and unrestricted Hartree-Fock computations gave conflicting answers. Burnham, in 1969, concluded that the trustworthiest computations supported ''meta'' attack; Birch and Radom, in 1980, concluded that both ''ortho'' and ''meta'' substitutions would occur with a slight preference for ''ortho''.
*
*
In the earlier 1990s, Zimmerman and Wang developed an experiment technique to distinguish between ''ortho'' and ''meta'' protonation. The method began with the premise that carbanions are much more basic than the corresponding radical anions and thus protonate less selectively. Correspondingly, the two protonations in Birch reduction should exhibit an isotope effect: in a protium–deuterium medium, the radical anion should preferentially protonate and the carbanion deuterate. Indeed, a variety of methoxylated aromatics exhibited less ''ortho'' deuterium than ''meta'' (a 1:7 ratio). Moreover, modern electron density computations now firmly indicated ''ortho'' protonation; frontier orbital densities, most analogous to the traditional computations used in past studies, did not.
Although Birch remained reluctant to concede that ''ortho'' protonation was preferred as late as 1996,See diagrams in:
*
*
Zimmerman and Wang had won the day: modern textbooks unequivocally agree that electron-donating substituents promote ''ortho'' attack.
For electron-donating substituents, Birch initially proposed ''meta'' attack, corresponding to the location of greatest electron density in a neutral benzene ring, a position endorsed by Krapcho and Bothner-By. These conclusions were challenged by Zimmerman in 1961, who computed electron densities of the radical and diene anions, revealing that the ''ortho'' site which was most negative and thus most likely to protonate. But the situation remained uncertain, because computations remained highly sensitive to transition geometry. Worse, Hückel orbital and unrestricted Hartree-Fock computations gave conflicting answers. Burnham, in 1969, concluded that the trustworthiest computations supported ''meta'' attack; Birch and Radom, in 1980, concluded that both ''ortho'' and ''meta'' substitutions would occur with a slight preference for ''ortho''.
*
*
In the earlier 1990s, Zimmerman and Wang developed an experiment technique to distinguish between ''ortho'' and ''meta'' protonation. The method began with the premise that carbanions are much more basic than the corresponding radical anions and thus protonate less selectively. Correspondingly, the two protonations in Birch reduction should exhibit an isotope effect: in a protium–deuterium medium, the radical anion should preferentially protonate and the carbanion deuterate. Indeed, a variety of methoxylated aromatics exhibited less ''ortho'' deuterium than ''meta'' (a 1:7 ratio). Moreover, modern electron density computations now firmly indicated ''ortho'' protonation; frontier orbital densities, most analogous to the traditional computations used in past studies, did not.
Although Birch remained reluctant to concede that ''ortho'' protonation was preferred as late as 1996,See diagrams in:
*
*
Zimmerman and Wang had won the day: modern textbooks unequivocally agree that electron-donating substituents promote ''ortho'' attack.
alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
(traditionally sodium) and a proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
source (traditionally an alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
). Unlike catalytic hydrogenation, Birch reduction does not reduce the aromatic ring all the way to a cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
.
naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromati ...
in ammonia and ethanol: 
Reaction mechanism and regioselectivity
A solution of sodium in liquid ammonia consists of the intensely blueelectride
An electride is an ionic compound in which an electron is the anion. Solutions of alkali metals in ammonia are electride salts. In the case of sodium, these blue solutions consist of a(NH3)6sup>+ and solvated electrons:
:Na + 6 NH3 → a(N ...
salt a(NH3)xsup>+ e−. The solvated electrons add to the aromatic ring to give a radical anion, which then abstracts a proton from the alcohol. The process then repeats at either the ''ortho'' or ''para'' position (depending on substituents) to give the final diene. The residual double bonds do not stabilize further radical additions.
The reaction is known to be third order – first order in the aromatic, first order in the alkali metal, and first order in the alcohol. This requires that the rate-limiting step be the conversion of radical anion B to the cyclohexadienyl radical C.
That step also determines the structure of the product. Although Arthur Birch originally argued that the protonation occurred at the ''meta'' position, subsequent investigation has revealed that protonation occurs at either the ''ortho'' or ''para'' position. Electron donors tend to induce ''ortho'' protonation, as shown in the reduction of anisole (1). Electron-withdrawing substituents tend to induce ''para'' protonation, as shown in the reduction of benzoic acid (2).
ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
or nitro groups, but do not attack alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is ...
, carboxylic acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
, or ethers.
Secondary protonation regioselectivity
The second reduction and protonation also poses mechanistic questions. Thus there are three resonance structures for the carbanion (labeled B, C and D in the picture).tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydr ...
) kinetically protonate, they do so at the center to afford the β,γ-unsaturated carbonyl.
Modifications
Traditional Birch reduction requirescryogenic
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cr ...
temperatures to liquify ammonia and pyrophoric alkali-metal electron donors. Variants have developed to reduce either inconvenience.
Many amines serve as alternative solvents: for example, THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is ma ...
or mixed ''n''-propylamine and ethylenediamine
Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately ...
.
To avoid direct alkali, there are chemical alternatives, such as M-SG reducing agent
In M-SG an alkali metal is absorbed into silica gel at elevated temperatures. The resulting black powder material is an effective reducing agent and safe to handle as opposed to the pure metal. The material can also be used as a desiccant and as a ...
. The reduction can also be powered by an external potential or sacrificial anode (magnesium or aluminum), but then alkali metal salts are necessary to colocate the reactants via complexation.
Birch alkylation
In Birch alkylation the anion formed in the Birch reduction is trapped by a suitable electrophile such as ahaloalkane
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely us ...
, for example:
:electron-withdrawing substituent
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of t ...
, such as a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
, will stabilize the carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3C ...
to generate the least-substituted olefin
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
; an electron-donating substituent
In chemistry, electron-rich is jargon that is used in multiple related meanings with either or both kinetic and thermodynamic implications:
*with regards to electron-transfer, electron-rich species have low ionization energy and/or are oxidation ...
has the opposite effect.
:
Benkeser reduction
The Benkeser reduction is the hydrogenation of polycyclic aromatic hydrocarbons, especiallynaphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromati ...
s using lithium or calcium metal in low molecular weight alkyl amines solvents. Unlike traditional Birch reduction, the reaction can be conducted at temperatures higher than the boiling point of ammonia (−33 °C).
For the reduction of naphthalene with lithium in a mixed ethylamine
Ethylamine, also known as ethanamine, is an organic compound with the formula CH3CH2NH2. This colourless gas has a strong ammonia-like odor. It condenses just below room temperature to a liquid miscible with virtually all solvents. It is a nucleo ...
- dimethylamine solution, the principal products are bicyclo .3.0ec-(1,9)-ene, bicyclo .3.0ec-(1,2)-ene and bicyclo .3.0ecane.
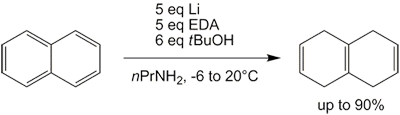 The directing effects of naphthalene substituents remain relatively unstudied theoretically. Substituents adjacent to the bridge appear to direct reduction to the unsubstituted ring; β substituents (one bond further) tend to direct reduction to the substituted ring.
The directing effects of naphthalene substituents remain relatively unstudied theoretically. Substituents adjacent to the bridge appear to direct reduction to the unsubstituted ring; β substituents (one bond further) tend to direct reduction to the substituted ring.
History
Arthur Birch, building on earlier work by Wooster and Godfrey, developed the reaction while working in theDyson Perrins Laboratory
The Dyson Perrins Laboratory is in the science area of the University of Oxford and was the main centre for research into organic chemistry of the University from its foundation in 1916 until its closure as a research laboratory in 2003. Until 2 ...
at the University of Oxford.
*
*
*
*
*
*
Birch's original procedure used sodium and ethanol; Alfred L. Wilds Alfred Lawrence Wilds (1 March 1915 - 4 July 2002) was a professor emeritus of chemistry at the University of Wisconsin in Madison.
He graduated at the University of Michigan in 1937 and earned a Ph.D. in 1939.
His doctoral research was done unde ...
later discovered that lithium gives better yields.
The reaction was difficult to understand mechanistically, with controversy lasting into the 1990s.
The case with electron-withdrawing groups is obvious, because the Birch alkylation serves as a trap for the penultimate dianion D. This dianion appears even in alcohol-free reactions. Thus the initial protonation is ''para'' rather than ''ipso'', as seen in the B-C transformation.
Additional reading
*See also
* Solvated electron — the reducing agent *Bouveault–Blanc reduction
The Bouveault–Blanc reduction is a chemical reaction in which an ester is reduced to primary alcohols using absolute ethanol and sodium metal. It was first reported by Louis Bouveault and Gustave Louis Blanc in 1903. Bouveault and Blanc demons ...
— another reaction using solvated electrons
* Synthesis of methamphetamine — an application
References
{{DEFAULTSORT:Birch Reduction Organic reduction reactions Organic redox reactions Name reactions