Benzoylindole Structure General on:
[Wikipedia]
[Google]
[Amazon]
To combat the illicit  Notice the five carbon chain with the fluorine atom attached to the nitrogen atom. A fluoropentyl chain falls within the scope of "haloalkyl" substitutions, and so with a methyl group attached to the carbon atom at the 4-position of the naphthyl ring (i.e. "substituted in the naphthyl ring to any extent"), and a fluoropentyl group attached to the nitrogen atom ("with substitution at the nitrogen atom of the indole ring by ..haloalkyl...group"), this compound falls within the scope of the general definition. It is in this way MAM-2201 can be controlled without being specifically named in the statute. On the other hand,
Notice the five carbon chain with the fluorine atom attached to the nitrogen atom. A fluoropentyl chain falls within the scope of "haloalkyl" substitutions, and so with a methyl group attached to the carbon atom at the 4-position of the naphthyl ring (i.e. "substituted in the naphthyl ring to any extent"), and a fluoropentyl group attached to the nitrogen atom ("with substitution at the nitrogen atom of the indole ring by ..haloalkyl...group"), this compound falls within the scope of the general definition. It is in this way MAM-2201 can be controlled without being specifically named in the statute. On the other hand,
 Phenylacetylindoles: Any compound containing a 3-phenylacetylindole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl,1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not further substituted in the indole ring to any extent and whether or not substituted in the phenyl ring to any extent.
One example given is
Phenylacetylindoles: Any compound containing a 3-phenylacetylindole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl,1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not further substituted in the indole ring to any extent and whether or not substituted in the phenyl ring to any extent.
One example given is  Benzoylindoles: Any compound containing a 3-(benzoyl)indole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not further substituted in the indole ring to any extent and whether or not substituted in the phenyl ring to any extent.
One example is
Benzoylindoles: Any compound containing a 3-(benzoyl)indole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not further substituted in the indole ring to any extent and whether or not substituted in the phenyl ring to any extent.
One example is  Cyclohexylphenols: Any compound containing a 2-(3-hydroxycyclohexyl)phenol structure with substitution at the 5-position of the phenolic ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not substituted in the cyclohexyl ring to any extent.
One example is
Cyclohexylphenols: Any compound containing a 2-(3-hydroxycyclohexyl)phenol structure with substitution at the 5-position of the phenolic ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not substituted in the cyclohexyl ring to any extent.
One example is  Naphthylmethylindoles: Any compound containing a 1H-indol-3-yl-(1-naphthyl)methane structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not further substituted in the indole ring to any extent and whether or not substituted in the naphthyl ring to any extent.
One example is
Naphthylmethylindoles: Any compound containing a 1H-indol-3-yl-(1-naphthyl)methane structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not further substituted in the indole ring to any extent and whether or not substituted in the naphthyl ring to any extent.
One example is  Naphthoylpyrroles: Any compound containing a 3-(1-naphthoyl)pyrrole structure with substitution at the nitrogen atom of the pyrrole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not further substituted in the pyrrole ring to any extent and whether or not substituted in the naphthyl ring to any extent.
One example is
Naphthoylpyrroles: Any compound containing a 3-(1-naphthoyl)pyrrole structure with substitution at the nitrogen atom of the pyrrole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not further substituted in the pyrrole ring to any extent and whether or not substituted in the naphthyl ring to any extent.
One example is  Naphthylmethylindenes: Any compound containing a 1-(1-naphthylmethyl)indene structure with substitution at the 3-position of the indene ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not further substituted in the indene ring to any extent and whether or not substituted in the naphthyl ring to any extent.
One example is JWH-176. Notice the 5 membered pentyl chain on the 3-position of the indene ring. Strictly speaking this chemical name is incorrect, as JWH-176 and related compounds would more correctly be viewed as derivatives of 1-(1-naphthyl methylylidene)indene due to the unsaturated =CH- linker group (as opposed to the -CH2- linker group found in e.g. naphthylmethylindoles), however "Naphthylmethylindenes" has gained acceptance as a legal term of art in this instance.
Naphthylmethylindenes: Any compound containing a 1-(1-naphthylmethyl)indene structure with substitution at the 3-position of the indene ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not further substituted in the indene ring to any extent and whether or not substituted in the naphthyl ring to any extent.
One example is JWH-176. Notice the 5 membered pentyl chain on the 3-position of the indene ring. Strictly speaking this chemical name is incorrect, as JWH-176 and related compounds would more correctly be viewed as derivatives of 1-(1-naphthyl methylylidene)indene due to the unsaturated =CH- linker group (as opposed to the -CH2- linker group found in e.g. naphthylmethylindoles), however "Naphthylmethylindenes" has gained acceptance as a legal term of art in this instance.
 Tetramethylcyclopropanoylindoles: Any compound containing a 3-(1-tetramethylcyclopropoyl)indole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group, whether or not further substituted in the indole ring to any extent and whether or not further substituted in the tetramethylcyclopropyl ring to any extent. While all known examples of compounds from this group have a 2,2,3,3-tetramethylcyclopropyl substituent, this definition would also encompass other
Tetramethylcyclopropanoylindoles: Any compound containing a 3-(1-tetramethylcyclopropoyl)indole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group, whether or not further substituted in the indole ring to any extent and whether or not further substituted in the tetramethylcyclopropyl ring to any extent. While all known examples of compounds from this group have a 2,2,3,3-tetramethylcyclopropyl substituent, this definition would also encompass other 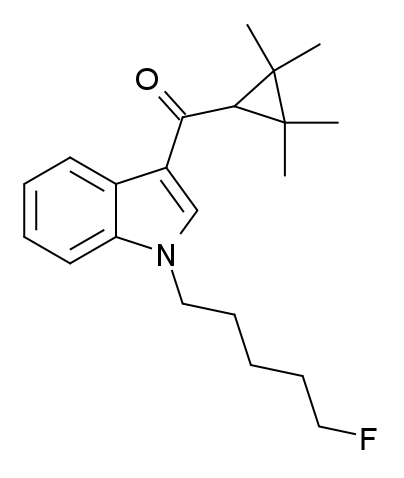 Adamantoylindoles: Any compound containing a 3-(1-adamantoyl)indole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl,or2-(4-morpholinyl)ethyl group, whether or not further substituted in the indole ring to any extent and whether or not substituted in the adamantyl ring system to any extent. Note that this definition (from the Kentucky statute) covers only compounds where the adamantyl group is attached by the 1-position, and would not include compounds where it is attached by the 2-position. Some other jurisdictions have consequently omitted the numbering from their corresponding definition (cf
Adamantoylindoles: Any compound containing a 3-(1-adamantoyl)indole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl,or2-(4-morpholinyl)ethyl group, whether or not further substituted in the indole ring to any extent and whether or not substituted in the adamantyl ring system to any extent. Note that this definition (from the Kentucky statute) covers only compounds where the adamantyl group is attached by the 1-position, and would not include compounds where it is attached by the 2-position. Some other jurisdictions have consequently omitted the numbering from their corresponding definition (cf
Arizona
for example), so as to cover a broader range of compounds. One example is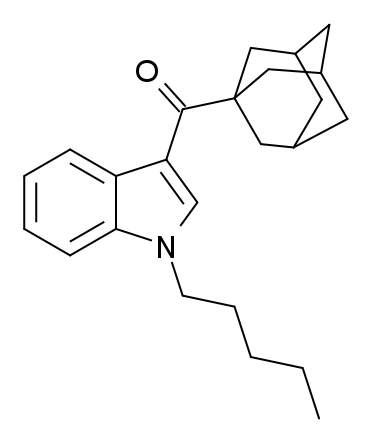 Indole-3-carboxylate esters: Any compound containing a 1H-indole-3-carboxylate ester structure with the ester oxygen bearing a napthyl, quinolinyl, isoquinolinyl, or adamantyl group and substitution at the one position of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, benzyl, N-methyl-2-piperidinylmethyl, or 2-(4-morpholinyl)ethyl group, whether or not further substituted on the indole ring to any extent and whether or not further substituted on the naphthyl, quinolinyl, isoquinolinyl, adamantyl, or benzyl groups to any extent.
One example is
Indole-3-carboxylate esters: Any compound containing a 1H-indole-3-carboxylate ester structure with the ester oxygen bearing a napthyl, quinolinyl, isoquinolinyl, or adamantyl group and substitution at the one position of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, benzyl, N-methyl-2-piperidinylmethyl, or 2-(4-morpholinyl)ethyl group, whether or not further substituted on the indole ring to any extent and whether or not further substituted on the naphthyl, quinolinyl, isoquinolinyl, adamantyl, or benzyl groups to any extent.
One example is  Indazole-3-carboxamides: Any compound containing a 1H-indazole-3-carboxamide structure with substitution at the nitrogen of the carboxamide by a naphthyl, quinolinyl, isoquinolinyl, adamantyl, or 1-amino-1-oxoalkan-2-yl group and substitution at the one position of the indazole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, benzyl, N-methyl-2-piperidinylmethyl, or 2-(4-morpholinyl)ethyl group, whether or not further substituted on the indazole ring to any extent and whether or not further substituted on the naphthyl, quinolinyl, isoquinolinyl, adamantyl, 1-amino-oxoalkan-2-yl, or benzyl groups to any extent.
One example is
Indazole-3-carboxamides: Any compound containing a 1H-indazole-3-carboxamide structure with substitution at the nitrogen of the carboxamide by a naphthyl, quinolinyl, isoquinolinyl, adamantyl, or 1-amino-1-oxoalkan-2-yl group and substitution at the one position of the indazole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, benzyl, N-methyl-2-piperidinylmethyl, or 2-(4-morpholinyl)ethyl group, whether or not further substituted on the indazole ring to any extent and whether or not further substituted on the naphthyl, quinolinyl, isoquinolinyl, adamantyl, 1-amino-oxoalkan-2-yl, or benzyl groups to any extent.
One example is 
 For example, the wording of this Texas statute encompasses a large range of "prophetic" core structures which have not yet been encountered in synthetic cannabinoids, but might plausibly be likely to appear in future (e.g. "Quinolinoyl pyrazole carboxylate", "Naphthoylimidazole" etc);
"(a) In this section:
(1) “Core component” is one of the following: azaindole,
For example, the wording of this Texas statute encompasses a large range of "prophetic" core structures which have not yet been encountered in synthetic cannabinoids, but might plausibly be likely to appear in future (e.g. "Quinolinoyl pyrazole carboxylate", "Naphthoylimidazole" etc);
"(a) In this section:
(1) “Core component” is one of the following: azaindole,
synthetic cannabinoid
Synthetic cannabinoids are a class of designer drug molecules that bind to the same receptors to which cannabinoids (THC, CBD and many others) in cannabis plants attach. These novel psychoactive substances should not be confused with synthetic ...
industry many jurisdictions have created a system to control these cannabinoids through their general (or Markush) structure as opposed to their specific identity. In this way new analogs are already controlled before they are even created. A large number of cannabinoids have been grouped into classes based on similarities in their chemical structure
A chemical structure determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target molecule or other solid. Molecular geometry refers to the spatial arrangement of at ...
, and these classes have been widely adopted across a variety of jurisdictions.
Typical groups of compounds included for control may include naphthoylindoles, phenylacetylindoles, benzoylindoles, cyclohexylphenols, naphthylmethylindoles, naphthoylpyrroles, naphthylmethylindenes, indole-3-carboxamides, indole-3-carboxylates, indazole-3-carboxamides and sometimes others, each with specific substitutions on specific atoms of the molecule. The scope of definitions and the range of compounds included may vary substantially between jurisdictions, so compounds which are legal in one country or state may be illegal in another.
For example, in many jurisdictions there is a general control on Naphthoylindoles: "Any compound containing a 3-(1- naphth oyl)indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environmen ...
structure with substitution at the nitrogen atom of the indole ring by an alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
, halo
Halo, halos or haloes usually refer to:
* Halo (optical phenomenon)
* Halo (religious iconography), a ring of light around the image of a head
HALO, halo, halos or haloes may also refer to:
Arts and entertainment Video games
* ''Halo'' (franch ...
alkyl, alkenyl
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
, cycloalkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
methyl, cycloalkylethyl, 1-(N-methyl-2- piperidinyl)methyl, or 2-(4- morpholinyl)ethyl group, whether or not further substituted in the indole ring to any extent and whether or not substituted in the naphthyl ring to any extent." (example definition from Kentucky, which is substantially derived from the 2009 ACMD advice on synthetic cannabinoids in the UK) This causes a substance such as MAM-2201
MAM-2201 (4'-methyl-AM-2201, 5"-fluoro-JWH-122) is a drug that presumably acts as a potent agonist for the cannabinoid receptors. It had never previously been reported in the scientific or patent literature, and was first identified by laborato ...
to be controlled as a Schedule 1 illegal drug, even though "MAM-2201" or its corresponding chemical name are not specifically listed in the statute.
 Notice the five carbon chain with the fluorine atom attached to the nitrogen atom. A fluoropentyl chain falls within the scope of "haloalkyl" substitutions, and so with a methyl group attached to the carbon atom at the 4-position of the naphthyl ring (i.e. "substituted in the naphthyl ring to any extent"), and a fluoropentyl group attached to the nitrogen atom ("with substitution at the nitrogen atom of the indole ring by ..haloalkyl...group"), this compound falls within the scope of the general definition. It is in this way MAM-2201 can be controlled without being specifically named in the statute. On the other hand,
Notice the five carbon chain with the fluorine atom attached to the nitrogen atom. A fluoropentyl chain falls within the scope of "haloalkyl" substitutions, and so with a methyl group attached to the carbon atom at the 4-position of the naphthyl ring (i.e. "substituted in the naphthyl ring to any extent"), and a fluoropentyl group attached to the nitrogen atom ("with substitution at the nitrogen atom of the indole ring by ..haloalkyl...group"), this compound falls within the scope of the general definition. It is in this way MAM-2201 can be controlled without being specifically named in the statute. On the other hand, THJ-2201
THJ-2201 is an indazole-based synthetic cannabinoid that presumably acts as a potent agonist of the CB1 receptor and has been sold online as a designer drug.
It is a structural analog of AM-2201 in which the central indole ring has been replac ...
with an indazole
Indazole, also called isoindazole, is a heterocyclic aromatic organic compound. This bicyclic compound consists of the fusion of benzene and pyrazole.
Indazole is an amphoteric molecule which can be protonated to an indazolium cation or deproton ...
core, falls outside this general definition, as it is a naphthoylindazole rather than a naphthoylindole. Note however that THJ-2201 is now specifically listed under US federal law.
Common examples of general controls
Naphthoylindoles: Any compound containing a 3-(1-naphthoyl)indole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group, whether or not further substituted in the indole ring to any extent and whether or not substituted in the naphthyl ring to any extent. One specific example given isJWH-018
JWH-018 (1-pentyl-3-(1-naphthoyl)indole, NA-PIMO or AM-678) is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in a ...
, one of the earliest synthetic cannabinoids identified. Notice the indole ring has an alkyl substitution on the nitrogen atom, but there are no additional substitutions elsewhere on the molecule.
JWH-250
JWH-250 or (1-pentyl-3-(2-methoxyphenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist at both the CB1 and CB2 receptors, with a ''K''i of 11 nM at CB1 and 33 nM at CB2. U ...
. There is an alkyl substitution on the nitrogen atom of the indole ring as well as a methoxy group attached to the phenyl ring.
RCS-4
RCS-4, or 1-pentyl-3-(4-methoxybenzoyl)indole, is a synthetic cannabinoid drug sold under the names SR-19, BTM-4, or Eric-4 (later shortened to E-4), but originally, OBT-199.
Pharmacology
RCS-4 is a potent cannabinoid receptor agonist, with EC ...
. Note the alkyl group substitution on the nitrogen atom of the indole. It is further substituted in the phenyl ring with a methoxy
In organic chemistry, a methoxy group is the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula .
On a benzene ring, the Hammett equation classifies a methoxy substituent at the ''para'' position as ...
group.
 Cyclohexylphenols: Any compound containing a 2-(3-hydroxycyclohexyl)phenol structure with substitution at the 5-position of the phenolic ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not substituted in the cyclohexyl ring to any extent.
One example is
Cyclohexylphenols: Any compound containing a 2-(3-hydroxycyclohexyl)phenol structure with substitution at the 5-position of the phenolic ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group whether or not substituted in the cyclohexyl ring to any extent.
One example is CP 47,497
CP 47,497 or (C7)-CP 47,497 is a cannabinoid receptor agonist drug, developed by Pfizer in the 1980s. It has analgesic effects and is used in scientific research. It is a potent CB1 agonist with a ''K''d of 2.1 nM.
Homologue
On the 19th of ...
. Notice the methyloctan-2-yl alkyl group substituted onto the 5-position of the phenol ring of the molecule. Note that this definition encompasses only those compounds that have OH groups attached to both the phenyl and the cyclohexyl rings, and so does not include compounds such as O-1871
O-1871 is a potent cannabinoid agonist which was invented by Billy R Martin and Raj K Razdan at Organix Inc in 2002. It has a CB1 receptor affinity of 2.0nM and a CB2 receptor affinity of 0.3nM. Structurally, O-1871 is a cyclohexylphenol deriva ...
which lacks the cyclohexyl OH group, or compounds such as JWH-337 or JWH-344 which lack the phenolic OH group. Some jurisdictions have addressed this by naming such compounds specifically, alternatively some have adopted broader definitions (such as in the Australian Federal Poisons Standard, which controls all derivatives of cyclohexylphenol unless otherwise specified).
JWH-175
JWH-175 is a drug from the naphthylmethylindole family which acts as a cannabinoid receptor agonist. It was invented by the scientist John W. Huffman and colleagues at Clemson University. JWH-175 is closely related to the widely used cannabinoi ...
. Notice the pentyl group substituted onto the nitrogen atom of the indole ring.
JWH-030
JWH-030 is a research chemical which is a cannabinoid receptor agonist. It has analgesic effects and is used in scientific research. It is a partial agonist at Cannabinoid receptor type 1, CB1 receptors, with a Dissociation constant, Ki of 87 ...
. Notice the pentyl group on the nitrogen atom of the pyrrole ring of the molecule.
isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
s.
One example is XLR-11
XLR-11 (5"-fluoro-UR-144 or 5F-UR-144) is a drug that acts as a potent agonist for the cannabinoid receptors CB1 and CB2 with EC50 values of 98 nM and 83 nM, respectively. It is a 3-(tetramethylcyclopropylmethanoyl)indole derivative related t ...
. Notice the 5 membered alkyl group ending with a fluorine atom substituted onto the nitrogen atom of the indole group.
 Adamantoylindoles: Any compound containing a 3-(1-adamantoyl)indole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl,or2-(4-morpholinyl)ethyl group, whether or not further substituted in the indole ring to any extent and whether or not substituted in the adamantyl ring system to any extent. Note that this definition (from the Kentucky statute) covers only compounds where the adamantyl group is attached by the 1-position, and would not include compounds where it is attached by the 2-position. Some other jurisdictions have consequently omitted the numbering from their corresponding definition (cf
Adamantoylindoles: Any compound containing a 3-(1-adamantoyl)indole structure with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl,or2-(4-morpholinyl)ethyl group, whether or not further substituted in the indole ring to any extent and whether or not substituted in the adamantyl ring system to any extent. Note that this definition (from the Kentucky statute) covers only compounds where the adamantyl group is attached by the 1-position, and would not include compounds where it is attached by the 2-position. Some other jurisdictions have consequently omitted the numbering from their corresponding definition (cfArizona
for example), so as to cover a broader range of compounds. One example is
AB-001
AB-001 (1-pentyl-3-(1-adamantoyl)indole) is a designer drug that was found as an ingredient in synthetic cannabis smoking blends in Ireland in 2010 and Hungary and Germany in 2011. It is unclear who AB-001 was originally developed by, but it is ...
. Notice the 5-membered alkyl group on the nitrogen atom of the indole ring.
 Indole-3-carboxylate esters: Any compound containing a 1H-indole-3-carboxylate ester structure with the ester oxygen bearing a napthyl, quinolinyl, isoquinolinyl, or adamantyl group and substitution at the one position of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, benzyl, N-methyl-2-piperidinylmethyl, or 2-(4-morpholinyl)ethyl group, whether or not further substituted on the indole ring to any extent and whether or not further substituted on the naphthyl, quinolinyl, isoquinolinyl, adamantyl, or benzyl groups to any extent.
One example is
Indole-3-carboxylate esters: Any compound containing a 1H-indole-3-carboxylate ester structure with the ester oxygen bearing a napthyl, quinolinyl, isoquinolinyl, or adamantyl group and substitution at the one position of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, benzyl, N-methyl-2-piperidinylmethyl, or 2-(4-morpholinyl)ethyl group, whether or not further substituted on the indole ring to any extent and whether or not further substituted on the naphthyl, quinolinyl, isoquinolinyl, adamantyl, or benzyl groups to any extent.
One example is PB-22
PB-22 (QUPIC, SGT-21 or 1-pentyl-1''H''-indole-3-carboxylic acid 8-quinolinyl ester) is a designer drug offered by online vendors as a cannabimimetic agent, and detected being sold in synthetic cannabis products in Japan in 2013. PB-22 represents ...
. Notice the quinolinyl group attached to the oxygen atom and the 5 carbon chain (pentyl) group on the Nitrogen atom.
 Indazole-3-carboxamides: Any compound containing a 1H-indazole-3-carboxamide structure with substitution at the nitrogen of the carboxamide by a naphthyl, quinolinyl, isoquinolinyl, adamantyl, or 1-amino-1-oxoalkan-2-yl group and substitution at the one position of the indazole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, benzyl, N-methyl-2-piperidinylmethyl, or 2-(4-morpholinyl)ethyl group, whether or not further substituted on the indazole ring to any extent and whether or not further substituted on the naphthyl, quinolinyl, isoquinolinyl, adamantyl, 1-amino-oxoalkan-2-yl, or benzyl groups to any extent.
One example is
Indazole-3-carboxamides: Any compound containing a 1H-indazole-3-carboxamide structure with substitution at the nitrogen of the carboxamide by a naphthyl, quinolinyl, isoquinolinyl, adamantyl, or 1-amino-1-oxoalkan-2-yl group and substitution at the one position of the indazole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, benzyl, N-methyl-2-piperidinylmethyl, or 2-(4-morpholinyl)ethyl group, whether or not further substituted on the indazole ring to any extent and whether or not further substituted on the naphthyl, quinolinyl, isoquinolinyl, adamantyl, 1-amino-oxoalkan-2-yl, or benzyl groups to any extent.
One example is AB-CHMINACA
AB-CHMINACA is an indazole-based synthetic cannabinoid. It is a potent agonist of the CB1 receptor (''K''i = 0.78 nM) and CB2 receptor (''K''i = 0.45 nM) and fully substitutes for Δ9-THC in rat discrimination stu ...
. Notice the indole group has a cyclohexylmethyl (a type of cycloalkylmethyl) group attached at the nitrogen atom. Also there is an 1-amino-1-oxoalkan-2-yl group (1-amino-3-methyl-1-oxobutan-2-yl in this instance) substituted on the nitrogen atom of the carboxamide group.
Other approaches to general controls
One consequence of the introduction of broad Markush structure bans, has been the appearance of compounds which have similar structures but technically fall outside the scope of the legal definitions (for instance containing anindazole
Indazole, also called isoindazole, is a heterocyclic aromatic organic compound. This bicyclic compound consists of the fusion of benzene and pyrazole.
Indazole is an amphoteric molecule which can be protonated to an indazolium cation or deproton ...
core instead of the proscribed indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environmen ...
, or a carboxamide linker in place of methanone), therefore resulting over time in a progressively increasing structural diversity of synthetic cannabinoids sold for illicit recreational use.
Some jurisdictions have attempted to introduce a broader scope of coverage by defining "head", "core", "linker" and "tail" groups which can be interchanged in any combination that fits within the definition, resulting in a much wider (but still usually finite) range of compounds being encompassed.
 For example, the wording of this Texas statute encompasses a large range of "prophetic" core structures which have not yet been encountered in synthetic cannabinoids, but might plausibly be likely to appear in future (e.g. "Quinolinoyl pyrazole carboxylate", "Naphthoylimidazole" etc);
"(a) In this section:
(1) “Core component” is one of the following: azaindole,
For example, the wording of this Texas statute encompasses a large range of "prophetic" core structures which have not yet been encountered in synthetic cannabinoids, but might plausibly be likely to appear in future (e.g. "Quinolinoyl pyrazole carboxylate", "Naphthoylimidazole" etc);
"(a) In this section:
(1) “Core component” is one of the following: azaindole, benzimidazole
Benzimidazole is a heterocyclic aromatic organic compound. This bicyclic compound may be viewed as fused rings of the aromatic compounds benzene and imidazole. It is a colorless solid.
Preparation
Benzimidazole is produced by condensation of o- ...
, benzothiazole
Benzothiazole is an aromatic heterocyclic compound with the chemical formula . It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivatives are found in commercial products or ...
, carbazole
Carbazole is an aromatic heterocyclic organic compound. It has a tricyclic structure, consisting of two six-membered benzene rings fused on either side of a five-membered nitrogen-containing ring. The compound's structure is based on the indole str ...
, imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole Diazole refers ...
, indane
Indane or indan is an organic compound with the formula C6H4(CH2)3. It is a colorless liquid hydrocarbon. It is a petrochemical, a bicyclic compound. It occurs at the level of about 0.1% in coal tar. It is usually produced by hydrogenation of ...
, indazole, indene
Indene is a flammable polycyclic hydrocarbon with chemical formula . It is composed of a benzene ring fused with a cyclopentene ring. This aromatic liquid is colorless although samples often are pale yellow. The principal industrial use of in ...
, indole, pyrazole
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
...
, pyrazolopyridine, pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a d ...
, or pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-meth ...
.
(2) “Group A component” is one of the following: adamantane
Adamantane is an organic compound with a formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the m ...
, benzene, cycloalkylmethyl, isoquinoline
Isoquinoline is a heterocyclic aromatic organic compound. It is a structural isomer of quinoline. Isoquinoline and quinoline are benzopyridines, which are composed of a benzene ring fused to a pyridine ring. In a broader sense, the term isoquin ...
, methylpiperazine, naphthalene, phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
, quinoline
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only sli ...
, tetrahydronaphthalene, tetramethylcyclopropane, amino oxobutane, amino dimethyl oxobutane, amino phenyl oxopropane, methyl methoxy oxobutane, methoxy dimethyl oxobutane, methoxy phenyl oxopropane, or an amino acid.
(3) “Link component” is one of the following functional groups: carboxamide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
, carboxylate
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an ion with negative charge.
Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,...; ''carboxylat ...
, hydrazide Hydrazides in organic chemistry are a class of organic compounds with the formula RNHNH2 where R is acyl (R'CO-), sulfonyl (R'SO2-), or phosphoryl (R'2P(O)-). Unlike hydrazine and alkylhydrazines, hydrazides are nonbasic owing to the inductive infl ...
, methanone (ketone), ethanone, methanediyl ( methylene bridge), or methine
In organic chemistry, a methine group or methine bridge is a trivalent functional group , derived formally from methane. It consists of a carbon atom bound by two single covalent bond, bonds and one double bond, where one of the single bonds is t ...
...
...(5) any compound containing a core component substituted at the 1-position to any extent, and substituted at the 3-position with a link component attached to a group A component, whether or not the core component or group A component are further substituted to any extent"
Another approach (here from the UK) is to list an example structure and then specify ways in which it can be modified by swapping various parts of the molecule with alternative substituent groups, for example;
"...any compound (not being clonitazene
Clonitazene is an opioid analgesic of approximately three times the potency of morphine. It is related to etonitazene
Etonitazene is an analgesic drug, first reported in 1957, that has been shown to have approximately 1,000 to 1,500 times the po ...
, etonitazene
Etonitazene is an analgesic drug, first reported in 1957, that has been shown to have approximately 1,000 to 1,500 times the potency of morphine in animals.
Because it is characterized by a strong dependency potential and a tendency to produce pr ...
, acemetacin
Acemetacin is a non-steroidal anti-inflammatory drug (NSAID) used for the treatment of osteoarthritis, rheumatoid arthritis, lower back pain, and relieving post-operative pain. It is manufactured by Merck KGaA under the tradename Emflex, and is a ...
, atorvastatin
Atorvastatin is a statin medication used to prevent cardiovascular disease in those at high risk and to treat abnormal lipid levels. For the prevention of cardiovascular disease, statins are a first-line treatment. It is taken by mouth.
Common ...
, bazedoxifene
Bazedoxifene, used as bazedoxifene acetate, is a medication for bone problems and possibly (pending more study) for cancer. It is a third-generation selective estrogen receptor modulator (SERM). Since late 2013 it has had U.S. FDA approval for ba ...
, indometacin
Indometacin, also known as indomethacin, is a nonsteroidal anti-inflammatory drug (NSAID) commonly used as a prescription medication to reduce fever, pain, stiffness
Stiffness is the extent to which an object resists deformation in response t ...
, losartan
Losartan, sold under the brand name Cozaar among others, is a medication used to treat high blood pressure (hypertension). It is in the angiotensin II receptor antagonist, angiotensin receptor blocker (ARB) family of medication, and is consider ...
, olmesartan
Olmesartan, sold under the trade name Benicar among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It is a reasonable initial treatment for high blood pressure. It is taken by mouth. Versio ...
, proglumetacin
Proglumetacin (usually as the maleate salt, trade names Afloxan, Protaxon and Proxil) is a nonsteroidal anti-inflammatory drug (NSAID). It is metabolized in the body to indometacin and proglumide
Proglumide (Milid) is a drug that inhibits ga ...
, telmisartan
Telmisartan, sold under the brand name Micardis among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It is a reasonable initial treatment for high blood pressure. It is taken by mouth. ...
, viminol
Viminol (marketed under the brandname Dividol) is an opioid analgesic developed by a team at the drug company Zambon in the 1960s. Viminol is based on the α-pyrryl-2-aminoethanol structure, unlike any other class of opioids.
Viminol has both a ...
, zafirlukast
Zafirlukast is an orally administered leukotriene receptor antagonist (LTRA) used for the chronic treatment of asthma. While zafirlukast is generally well tolerated, headache and stomach upset often occur. Some rare side effects can occur, which ...
or a compound for the time being specified in sub-paragraphs (h) to (s) above) structurally related to 1-pentyl-3-(1-naphthoyl)indole (JWH-018), in that the four sub-structures, that is to say the indole ring, the pentyl substituent, the methanone linking group and the naphthyl ring, are linked together in a similar manner, whether or not any of the sub-structures have been modified, and whether or not substituted in any of the linked sub-structures with one or more univalent substituents and, where any of the sub-structures have been modified, the modifications of the sub-structures are limited to any of the following, that is to say -
#replacement of the indole ring with indane, indene, indazole, pyrrole, pyrazole, imidazole, benzimidazole, pyrrolo ,3-byridine, pyrrolo,2-c
The comma is a punctuation mark that appears in several variants in different languages. It has the same shape as an apostrophe or single closing quotation mark () in many typefaces, but it differs from them in being placed on the baseline o ...
yridine or pyrazolo ,4-byridine;
#replacement of the pentyl substituent with alkyl, alkenyl, benzyl, cycloalkylmethyl, cycloalkylethyl, (N-methylpiperidin-2-yl)methyl, 2-(4-morpholinyl)ethyl or (tetrahydropyran-4-yl)methyl;
#replacement of the methanone linking group with an ethanone, carboxamide, carboxylate, methylene bridge or methine group;
#replacement of the 1-naphthyl ring with 2-naphthyl, phenyl, benzyl, adamantyl, cycloalkyl, cycloalkylmethyl, cycloalkylethyl, bicyclo .2.1eptanyl, 1,2,3,4-tetrahydronaphthyl, quinolinyl, isoquinolinyl, 1-amino-1-oxopropan-2-yl, 1-hydroxy-1-oxopropan-2-yl, piperidinyl, morpholinyl, pyrrolidinyl, tetrahydropyranyl or piperazinyl."
Broadly worded controls such as above may inadvertently include large numbers of compounds which merely happen to have some structural similarity, but do not have similar pharmacological effects to the prohibited cannabinoid drugs. One approach to this is to specifically list examples of such compounds that need to be exempted from the general control, so that commonly used medicines do not become subject to control as illegal drug analogs. Another issue is that even with such a wide range of structural modifications covered, modern predictive drug discovery techniques may generate novel analogues which still fall outside the specified range of prohibited structures.
See also
*Comparison of phytocannabinoids
Cannabinoids () are compounds found in the cannabis plant or synthetic compounds that can interact with the endocannabinoid system. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (Delta-9-THC), the primary intoxica ...
References
{{Reflist Cannabinoids