BCDMH on:
[Wikipedia]
[Google]
[Amazon]
1-Bromo-3-chloro-5,5-dimethylhydantoin (BCDMH or bromochlorodimethylhydantoin) is a chemical structurally related to
PubChem Public Chemical Database (nih.gov)
External MSDS
Disinfectants Organobromides Organochlorides Hydantoins
hydantoin
Hydantoin, or glycolylurea, is a heterocyclic organic compound with the formula CH2C(O)NHC(O)NH. It is a colorless solid that arises from the reaction of glycolic acid and urea. It is an oxidized derivative of imidazolidine. In a more general sen ...
. It is a white crystalline compound with a slight bromine and acetone odor and is insoluble in water, but soluble in acetone.
BCDMH is an excellent source of both chlorine and bromine as it reacts slowly with water releasing hypochlorous acid and hypobromous acid. It used as a chemical disinfectant
A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than st ...
for recreational water sanitation and drinking water purification. BCDMH works in the following manner:
The initial BCDMH reacts with water (R = Dimethylhydantoin):
: BrClR + 2 H2O → HOBr + HOCl
Hypochlorous acid (HClO, HOCl, or ClHO) is a weak acid that forms when chlorine dissolves in water, and itself partially dissociates, forming hypochlorite, ClO−. HClO and ClO− are oxidizers, and the primary disinfection agents of chlorine solu ...
+ RH2
Hypobromous acid partially dissociates in water:
: HOBr → H+ + OBr−
Hypobromous acid oxidizes the substrate, itself being reduced to bromide:
: HOBr + Live pathogens → Br− + Dead pathogens
The bromide ions are oxidized with the hypochlorous acid that was formed from the initial BCDMH:
: Br− + HOCl → HOBr + Cl−
This produces more hypobromous acid; the hypochlorous acid itself act directly as a disinfectant in the process.
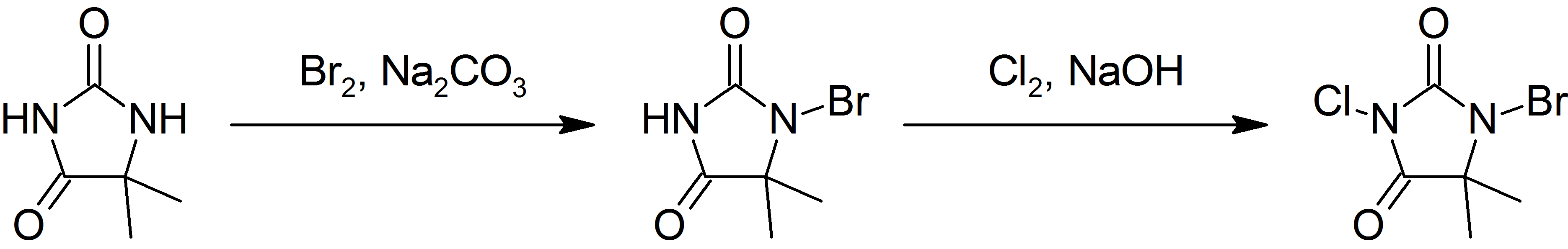
Preparation
This compound is prepared by first brominating, then chlorinating 5,5-dimethylhydantoin: :
References
{{ReflistExternal links
PubChem Public Chemical Database (nih.gov)
External MSDS
Disinfectants Organobromides Organochlorides Hydantoins