Aryne on:
[Wikipedia]
[Google]
[Amazon]
Arynes and benzynes are highly reactive species derived from an

 There are two possible regioisomers of benzyne with substituent (Y): triple bond can be positioned between C2 and C3 or between C3 and C4. Substituents ortho to the leaving group will lead to the triple bond between C2 and C3. Para Y and LG will lead to regioisomer with triple bond between C3 and C4. Meta substituent can afford both regioisomers as described above.
In case of triple bond located between C2 and C3, electron withdrawing (EWG) substituents, e.g. CF3, will direct the nucleophile addition to place carbanion as close as possible to EWG. However, electron donating (EDG) substituents, e.g. CH3, will provide little selectivity between products.
In the regioisomer where triple bond is located between C3 and C4 the effect of substituent on nucleophile addition is diminished, and mixtures of para and meta products are often obtained.
There are two possible regioisomers of benzyne with substituent (Y): triple bond can be positioned between C2 and C3 or between C3 and C4. Substituents ortho to the leaving group will lead to the triple bond between C2 and C3. Para Y and LG will lead to regioisomer with triple bond between C3 and C4. Meta substituent can afford both regioisomers as described above.
In case of triple bond located between C2 and C3, electron withdrawing (EWG) substituents, e.g. CF3, will direct the nucleophile addition to place carbanion as close as possible to EWG. However, electron donating (EDG) substituents, e.g. CH3, will provide little selectivity between products.
In the regioisomer where triple bond is located between C3 and C4 the effect of substituent on nucleophile addition is diminished, and mixtures of para and meta products are often obtained.

 A classic example is the synthesis of 1,2,3,4-tetraphenylnaphthalene. Tetrabromobenzene can react with
A classic example is the synthesis of 1,2,3,4-tetraphenylnaphthalene. Tetrabromobenzene can react with  +2cycloadditions of arynes have been commonly applied to natural product total synthesis. The main limitation of such approach, however, is the need to use constrained dienes, such as furan and cyclopentadiene. In 2009 Buszek and co-workers synthesized herbindole A using aryne +2cycloaddition. 6,7-indolyne undergoes +2cycloaddition with cyclopentadiene to afford complex tetracyclic product.
Benzynes undergo +2cycloaddition with a wide range of alkenes. Due to electrophilic nature of benzyne, alkenes bearing electron-donating substituents work best for this reaction.
Due to significant byproduct formation, aryne +2chemistry is rarely utilized in natural product total synthesis. Nevertheless, several examples do exist. In 1982, Stevens and co-workers reported a synthesis of taxodione that utilized +2cycloaddition between an aryne and a ketene acetal.
Mori and co-workers performed a palladium-catalyzed +2+2cocyclization of aryne and diyne in their total synthesis of taiwanins C.
+2cycloadditions of arynes have been commonly applied to natural product total synthesis. The main limitation of such approach, however, is the need to use constrained dienes, such as furan and cyclopentadiene. In 2009 Buszek and co-workers synthesized herbindole A using aryne +2cycloaddition. 6,7-indolyne undergoes +2cycloaddition with cyclopentadiene to afford complex tetracyclic product.
Benzynes undergo +2cycloaddition with a wide range of alkenes. Due to electrophilic nature of benzyne, alkenes bearing electron-donating substituents work best for this reaction.
Due to significant byproduct formation, aryne +2chemistry is rarely utilized in natural product total synthesis. Nevertheless, several examples do exist. In 1982, Stevens and co-workers reported a synthesis of taxodione that utilized +2cycloaddition between an aryne and a ketene acetal.
Mori and co-workers performed a palladium-catalyzed +2+2cocyclization of aryne and diyne in their total synthesis of taiwanins C.
 The interconversion of the 1,2-, 1,3- and 1,4-didehydrobenzenes has been studied. A 1,2- to 1,3-didehydrobenzene conversion has been postulated to occur in the
The interconversion of the 1,2-, 1,3- and 1,4-didehydrobenzenes has been studied. A 1,2- to 1,3-didehydrobenzene conversion has been postulated to occur in the

 Wittig and Pohmer found that benzyne participate in +2cycloaddition reactions.
Wittig and Pohmer found that benzyne participate in +2cycloaddition reactions.
 Additional evidence for the existence of benzyne came from spectroscopic studies. Benzyne has been observed in a "molecular container".
In 2015, a single aryne molecule was imaged by STM.
1,3-Didehydroarenes was first demonstrated in the 1990s when it was generated from 1,3-disubstituted benzene derivatives, such as the peroxy ester 1,3-C6H4(O2C(O)CH3)2.
Breakthroughs on 1,4-didehydrobenzene came in the 1960s, followed from studies on the
Additional evidence for the existence of benzyne came from spectroscopic studies. Benzyne has been observed in a "molecular container".
In 2015, a single aryne molecule was imaged by STM.
1,3-Didehydroarenes was first demonstrated in the 1990s when it was generated from 1,3-disubstituted benzene derivatives, such as the peroxy ester 1,3-C6H4(O2C(O)CH3)2.
Breakthroughs on 1,4-didehydrobenzene came in the 1960s, followed from studies on the
aromatic ring
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturat ...
by removal of two substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''side ...
s. Arynes are examples of didehydroarenes (1,2-didehydroarenes in this case), although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of strained alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s.
Bonding in arynes
The alkyne representation of benzyne is the most widely encountered. Arynes are usually described as having a strained triple bond. Geometric constraints on the triple bond in benzyne result in diminished overlap of in-plane p-orbitals, and thus weaker triple bond. The vibrational frequency of the triple bond in benzyne was assigned by Radziszewski to be 1846 cm−1, indicating a weaker triple bond than in unstrained alkyne with vibrational frequency of approximately 2150 cm−1. Nevertheless, benzyne is more like a strained alkyne than a diradical, as seen from the large singlet–triplet gap and alkyne-like reactivity. TheLUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
of aryne lies much lower than the LUMO of unstrained alkynes, which makes it a better energy match for the HOMO of nucleophiles. Hence, benzyne possesses electrophilic character and undergoes reactions with nucleophiles. A detailed MO analysis of benzyne was presented in 1968.
Generation of arynes
Due to their extreme reactivity, arynes must be generated ''in situ''. Typical of otherreactive intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these comp ...
s, benzyne must be trapped, otherwise it dimerises to biphenylene
Biphenylene is an organic compound with the formula (C6H4)2. It is a pale, yellowish solid with a hay-like odor. Despite its unusual structure, it behaves like a traditional polycyclic aromatic hydrocarbon.
Bonding
Biphenylene is a polycyclic hy ...
.
Early routes to benzyne involved dehydrohalogenation
In chemistry, dehydrohalogenation is an elimination reaction which removes a hydrogen halide from a substrate. The reaction is usually associated with the synthesis of alkenes, but it has wider applications.
Dehydrohalogenation from alkyl halid ...
of aryl halides In organic chemistry, an aryl halide (also known as haloarene) is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhi ...
:
Such reactions require strong base and high temperatures. 1,2-Disubstituted arenes serve as precursors to benzynes under milder conditions.
Benzyne is generated by the dehalogenation of 1-bromo-2-fluorobenzene by magnesium. Anthranilic acid
Anthranilic acid is an aromatic acid with the formula C6H4(NH2)(CO2H) and has a sweetish taste. The molecule consists of a benzene ring, ''ortho''-substituted with a carboxylic acid and an amine. As a result of containing both acidic and basic f ...
can be converted to 2-diazoniobenzene-1-carboxylate by diazotization and neutralization. Although explosive, this zwitterion
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively- and negatively-charged functional groups.
: With amino acids, for example, in solution a chemical equilibrium wil ...
ic species is a convenient and inexpensive precursor to benzyne.
Another method is based on trimethylsilylaryl triflate
In organic chemistry, triflate (systematic name: trifluoromethanesulfonate), is a functional group with the formula and structure . The triflate group is often represented by , as opposed to −Tf, which is the triflyl group, . For example, ' ...
s. Fluoride displacement of the trimethylsilyl group induces elimination of triflate and release of benzyne:
A hexadehydro Diels-Alder reaction (HDDA) involves cycloaddition of 1,3-diyne and alkyne.
''N''-amination of 1''H''-benzotriazole with hydroxylamine-''O''-sulfonic acid generates an intermediate which can be oxidised to benzyne in almost quantitative yield with lead(IV) acetate
Lead(IV) acetate or lead tetraacetate is an organometallic compound with chemical formula . It is a colorless solid that is soluble in nonpolar, organic solvents, indicating that it is not a salt. It is degraded by moisture and is typically store ...
.

Reactions of arynes
Even at low temperatures arynes are extremely reactive. Their reactivity can be classified in three main classes: (1) nucleophilic additions, (2) pericyclic reactions, and (3) bond-insertion.Nucleophilic additions to arynes
Upon treatment with basic nucleophiles, aryl halides deprotonate alpha to the leaving group, resulting indehydrohalogenation
In chemistry, dehydrohalogenation is an elimination reaction which removes a hydrogen halide from a substrate. The reaction is usually associated with the synthesis of alkenes, but it has wider applications.
Dehydrohalogenation from alkyl halid ...
. Isotope exchange studies indicate that for aryl fluorides and, sometimes, aryl chlorides, the elimination event proceeds in two steps, deprotonation, followed by expulsion of the nucleophile. Thus, the process is formally analogous to the E1cb mechanism of aliphatic compounds. Aryl bromides and iodides, on the other hand, generally appear to undergo elimination by a concerted syn-coplanar E2 mechanism. The resulting benzyne forms addition products, usually by nucleophilic addition and protonation. Generation of the benzyne intermediate is the slow step in the reaction.Anslyn, E. V.; Dougherty, D. A. ''Modern Physical Organic Chemistry.'' University Science Books, 2006
"Aryne coupling" reactions allow for generation of biphenyl compounds which are valuable in pharmaceutical industry, agriculture and as ligands in many metal-catalyzed transformations.
The metal–arene product can also add to another aryne, leading to chain-growth polymerization
Chain-growth polymerization (American English, AE) or chain-growth polymerisation (British English, BE) is a polymerization technique where Unsaturated compound, unsaturated monomer molecules add onto the active site on a growing polymer chain one ...
. Using copper(I) cyanide
Copper(I) cyanide is an inorganic compound with the formula CuCN. This off-white solid occurs in two polymorphs; impure samples can be green due to the presence of Cu(II) impurities. The compound is useful as a catalyst, in electroplating copper ...
as the initiator to add to the first aryne yielded polymers containing up to about 100 arene units.
When leaving group (LG) and substituent (Y) are mutually ortho or para, only one benzyne intermediate is possible. However, when LG is meta to Y, then regiochemical outcomes (A and B) are possible. If Y is electron withdrawing, then HB is more acidic than HA resulting in regioisomer B being generated. Analogously, if Y is electron donating, regioisomer A is generated, since now HA is the more acidic proton.
 There are two possible regioisomers of benzyne with substituent (Y): triple bond can be positioned between C2 and C3 or between C3 and C4. Substituents ortho to the leaving group will lead to the triple bond between C2 and C3. Para Y and LG will lead to regioisomer with triple bond between C3 and C4. Meta substituent can afford both regioisomers as described above.
In case of triple bond located between C2 and C3, electron withdrawing (EWG) substituents, e.g. CF3, will direct the nucleophile addition to place carbanion as close as possible to EWG. However, electron donating (EDG) substituents, e.g. CH3, will provide little selectivity between products.
In the regioisomer where triple bond is located between C3 and C4 the effect of substituent on nucleophile addition is diminished, and mixtures of para and meta products are often obtained.
There are two possible regioisomers of benzyne with substituent (Y): triple bond can be positioned between C2 and C3 or between C3 and C4. Substituents ortho to the leaving group will lead to the triple bond between C2 and C3. Para Y and LG will lead to regioisomer with triple bond between C3 and C4. Meta substituent can afford both regioisomers as described above.
In case of triple bond located between C2 and C3, electron withdrawing (EWG) substituents, e.g. CF3, will direct the nucleophile addition to place carbanion as close as possible to EWG. However, electron donating (EDG) substituents, e.g. CH3, will provide little selectivity between products.
In the regioisomer where triple bond is located between C3 and C4 the effect of substituent on nucleophile addition is diminished, and mixtures of para and meta products are often obtained.

Pericyclic reactions of arynes
Benzyne undergoes rapid dimerization to form biphenylene. Some routes to benzyne lead to especially rapid and high yield of this subsequent reaction.Trimerization
In chemistry, a trimer (; ) is a molecule or polyatomic anion formed by combination or association of three molecules or ions of the same substance. In technical jargon, a trimer is a kind of oligomer derived from three identical precursors often ...
gives triphenylene
Triphenylene is an organic compound with the formula (C6H4)3. A flat polycyclic aromatic hydrocarbon (PAH), it consists of four fused benzene rings. Triphenylene has delocalized 18-''π''-electron systems based on a planar structure, correspondin ...
.
Benzynes can undergo +2cyclization reactions. When generated in the presence of anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the Economic production, production of the red dye alizarin and other dyes ...
, trypticene results. In this method, the concerted mechanism of the Diels-Alder reaction between benzyne and furan is shown below. Other benzyne +2cycloadditions are thought to proceed via a stepwise mechanism.
butyllithium Butyllithium may refer to one of 5 isomeric organolithium reagents of which 3 are commonly used in chemical synthesis:
* ''n''-Butyllithium, abbreviated BuLi or nBuLi
* ''sec''-Butyllithium, abbreviated ''sec''-BuLi or sBuLi, has 2 stereoisomers, ...
and furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highly ...
to form a tetrahydroanthracene
Bond-insertion reactions of arynes
The first example of aryne σ-bond insertion reaction is the synthesis of melleine in 1973.Other dehydrobenzenes
If benzyne is 1,2-didehydrobenzene, two further isomers are possible: 1,3-didehydrobenzene and 1,4-didehydrobenzene. Their energiesin silico
In biology and other experimental sciences, an ''in silico'' experiment is one performed on computer or via computer simulation. The phrase is pseudo-Latin for 'in silicon' (correct la, in silicio), referring to silicon in computer chips. It ...
are, respectively, 106, 122, and 138 kcal/mol (444, 510 and 577 kJ/mol). The 1,2- and 1,3- isomers have singlet ground states, whereas for 1,4-didehydrobenzene the gap is smaller.
pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''py ...
(900 °C) of the phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
substituted aryne precursors as shown below. Extremely high temperatures are required for benzyne interconversion.
1,4-Didehydroarenes
In classical 1,4-didehydrobenzene experiments, heating to 300 °C, ,6-D2A readily equilibrates with ,2-D2B, but does not equilibrate with C or D. The simultaneous migration of deuterium atoms to form B, and the fact that none of C or D is formed can only be explained by a presence of a cyclic and symmetrical intermediate–1,4-didehydrobenzene. Two states were proposed for 1,4-didehydrobenzene: singlet and triplet, with the singlet state lower in energy. Triplet state represents two noninteracting radical centers, and hence should abstract hydrogens at the same rate as phenyl radical. However, singlet state is more stabilized than the triplet, and therefore some of the stabilizing energy will be lost in order to form the transition state for hydrogen cleavage, leading to slower hydrogen abstraction. Chen proposed the use of 1,4-didehydrobenzene analogues that have large singlet-triplet energy gaps to enhance selectivity of enediyne drug candidates.History
The first evidence for arynes came from the work of Stoermer and Kahlert. In 1902 they observed that upon treatment of 3-bromobenzofuran with base in ethanol 2-ethoxybenzofuran is formed. Based on this observation they postulated an aryne intermediate.Wittig Wittig is a surname, and may refer to:
* Burghardt Wittig (born 1947), German biochemist
* Curt Wittig, American chemist
* David Wittig (born 1955), American executive
* Edward Wittig (1879–1941), Polish sculptor
* Ferdinand Wittig (1851-1909 ...
et al. invoked zwitterionic intermediate in the reaction of fluorobenzene and phenyllithium to give biphenyl. This hypothesis was later confirmed.
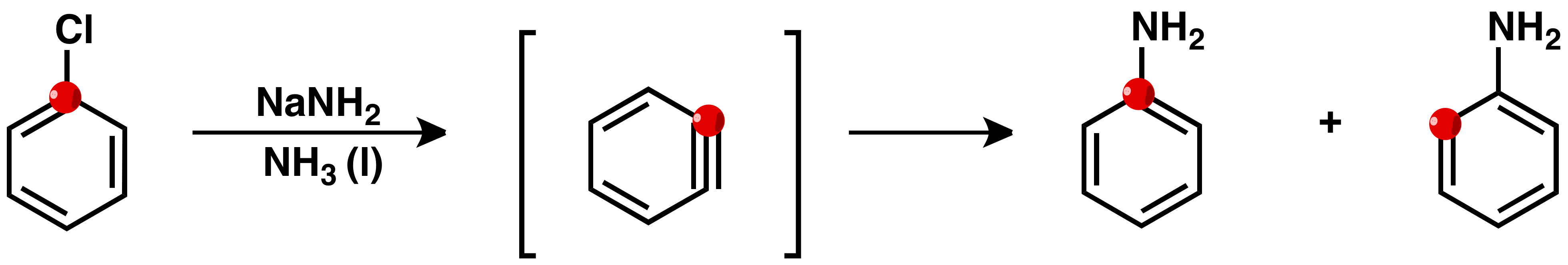
In 1953 14C labeling experiments provided strong support for the intermediacy of benzyne. John D. Roberts
John Dombrowski Roberts (June 8, 1918 – October 29, 2016) was an American chemist. He made contributions to the integration of physical chemistry, spectroscopy, and organic chemistry for the understanding of chemical reaction rates. Another ...
et al. showed that the reaction of chlorobenzene-1-14C and potassium amide gave equal amounts of aniline with 14C incorporation at C-1 and C-2.
 Wittig and Pohmer found that benzyne participate in +2cycloaddition reactions.
Wittig and Pohmer found that benzyne participate in +2cycloaddition reactions.
Bergman cyclization
The Masamune-Bergman cyclization or Masamune-Bergman reaction or Masamune-Bergman cycloaromatization is an organic reaction and more specifically a rearrangement reaction taking place when an enediyne is heated in presence of a suitable hydrogen do ...
. This theme became topical with the discovery of enediyne "cytostatics", such as calicheamicin
The calicheamicins are a class of enediyne antitumor antibiotics derived from the bacterium ''Micromonospora echinospora'', with calicheamicin γ1 being the most notable. It was isolated originally in the mid-1980s from the chalky soil, or "calich ...
, which generates a 1,4-didehydrobenzene.
Examples of benzynes in total synthesis
A variety of natural products have been prepared using arynes as intermediates. Nucleophilic additions to arynes have been widely used in natural product total synthesis. Indeed, nucleophilic additions of arynes are some of the oldest known applications of aryne chemistry. Nucleophilic addition to aryne was used in the attempted synthesis of cryptaustoline (1) and cryptowoline (2). The synthesis of the tetracyclic meroterpenoid (+)-liphagal involved an aryne intermediate. Their approach employed an aryne cyclization to close the final ring of the natural product. Multicomponent reactions of arynes are powerful transformations that allow for rapid formation of 1,2-disubsituted arenes. Despite their potential utility, examples of multicomponent aryne reactions in natural product synthesis are scarce. A four-component aryne coupling reaction was employed in the synthesis of dehydroaltenuene B.See also
* More examples use of aryne chemistry:tricyclobutabenzene
Tricyclobutabenzene is an aromatic hydrocarbon consisting of a benzene core with three cyclobutane rings fused onto it. This compound and related compounds are studied in the laboratory because they are often displaying unusual conformations and ...
, in-methylcyclophane, Transition metal benzyne complex
* The pyridine equivalent pyridyne
Pyridyne in chemistry is the pyridine analogue of benzyne. Pyridynes are the class of reactive intermediates derived from pyridine. Two isomers exist, the 2,3-pyridine (2,3-didehydropyridine) and the 3,4-pyridyne (3,4-didehydropyridine). The rea ...
References
External links
* {{Authority control Reactive intermediates Alkyne derivatives Aromatic hydrocarbons