Acid–base Reaction on:
[Wikipedia]
[Google]
[Amazon]
An acid–base reaction is a
chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
that occurs between an acid and a base. It can be used to determine pH via titration
Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to be analyzed). A reagent, termed the ''titrant'' ...
. Several theoretical
A theory is a rational type of abstract thinking about a phenomenon, or the results of such thinking. The process of contemplative and rational thinking is often associated with such processes as observational study or research. Theories may be ...
frameworks provide alternative conceptions of the reaction mechanisms and their application in solving related problems; these are called the acid–base theories, for example, Brønsted–Lowry acid–base theory
The Brønsted–Lowry theory (also called proton theory of acids and bases) is an acid–base reaction theory which was proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923. The fundamental concept of this the ...
.
Their importance becomes apparent in analyzing acid–base reactions for gaseous or liquid species, or when acid or base character may be somewhat less apparent. The first of these concepts was provided by the French chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties. Chemists carefully describe t ...
Antoine Lavoisier
Antoine-Laurent de Lavoisier ( , ; ; 26 August 17438 May 1794),
CNRS (
CNRS (
– Table of discoveries attributes Antoine Lavoisier as the first to posit a scientific theory in relation to
 The first modern definition of acids and bases in molecular terms was devised by
The first modern definition of acids and bases in molecular terms was devised by
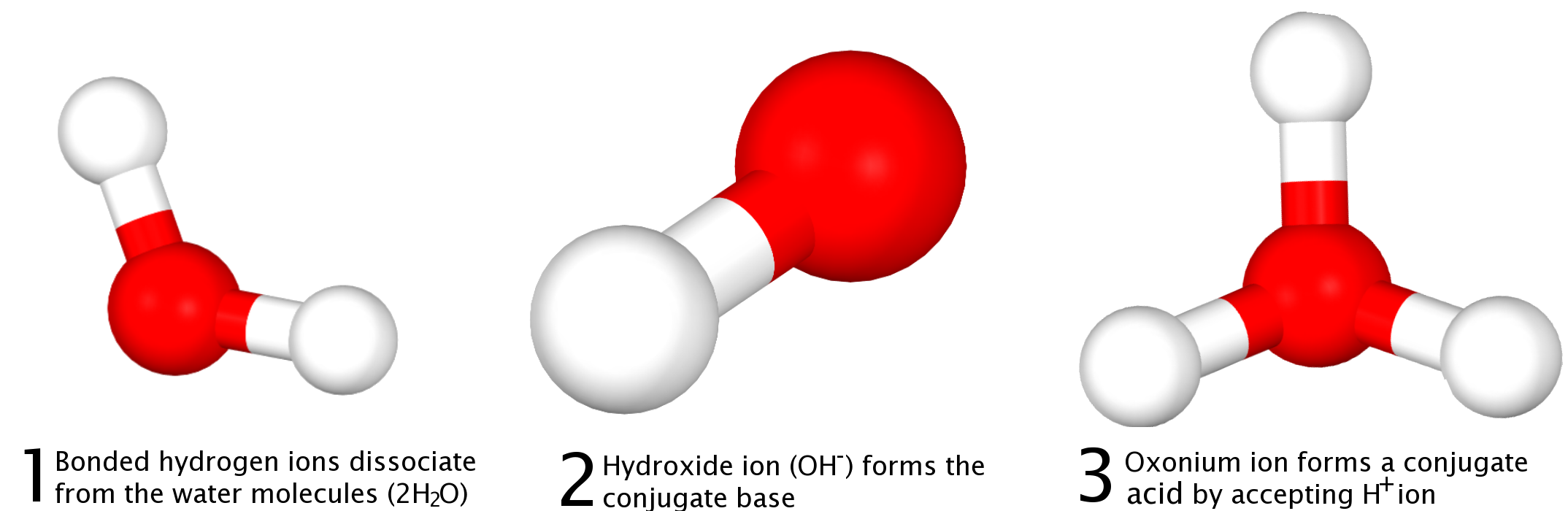 Here, one molecule of water acts as an acid, donating an H+ and forming the conjugate base, OH−, and a second molecule of water acts as a base, accepting the H+ ion and forming the conjugate acid, H3O+.
As an example of water acting as an acid, consider an aqueous solution of
Here, one molecule of water acts as an acid, donating an H+ and forming the conjugate base, OH−, and a second molecule of water acts as a base, accepting the H+ ion and forming the conjugate acid, H3O+.
As an example of water acting as an acid, consider an aqueous solution of
Acid-base Physiology: an on-line text
{{DEFAULTSORT:Acid-Base Reaction Acids Bases (chemistry) Acid-base chemistry Equilibrium chemistry
oxyacid
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce ...
s.
It is important to think of the acid-base reaction models as theories that complement each other. For example, the current Lewis model has the broadest definition of what an acid and base are, with the Brønsted-Lowry theory being a subset of what acids and bases are, and the Arrhenius theory being the most restrictive.
Acid–base definitions
Historic development
The concept of an acid-base reaction was first proposed in 1754 by Guillaume-François Rouelle, who introduced the word " base" into chemistry to mean a substance which reacts with an acid to give it solid form (as a salt). Bases are mostly bitter in nature.Lavoisier's oxygen theory of acids
The first scientific concept of acids and bases was provided by Lavoisier in around 1776. Since Lavoisier's knowledge ofstrong acid
Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions ...
s was mainly restricted to oxoacid
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce ...
s, such as (nitric acid) and (sulfuric acid), which tend to contain central atoms in high oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
s surrounded by oxygen, and since he was not aware of the true composition of the hydrohalic acid
In chemistry, hydrogen halides (hydrohalic acids when in the aqueous phase) are diatomic, inorganic compounds that function as Arrhenius acids. The formula is HX where X is one of the halogens: fluorine, chlorine, bromine, iodine, or astatine. A ...
s ( HF, HCl, HBr, and HI), he defined acids in terms of their containing ''oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
'', which in fact he named from Greek words meaning "acid-former" (from the Greek
Greek may refer to:
Greece
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group.
*Greek language, a branch of the Indo-European language family.
**Proto-Greek language, the assumed last common ancestor ...
ὀξύς (''oxys'') meaning "acid" or "sharp" and γεινομαι (''geinomai'') meaning "engender"). The Lavoisier definition held for over 30 years, until the 1810 article and subsequent lectures by Sir Humphry Davy
Sir Humphry Davy, 1st Baronet, (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several elements for the ...
in which he proved the lack of oxygen in , H2Te, and the hydrohalic acid
In chemistry, hydrogen halides (hydrohalic acids when in the aqueous phase) are diatomic, inorganic compounds that function as Arrhenius acids. The formula is HX where X is one of the halogens: fluorine, chlorine, bromine, iodine, or astatine. A ...
s. However, Davy failed to develop a new theory, concluding that "acidity does not depend upon any particular elementary substance, but upon peculiar arrangement of various substances". One notable modification of oxygen theory was provided by Jöns Jacob Berzelius, who stated that acids are oxides of nonmetals while bases are oxides of metals.
Liebig's hydrogen theory of acids
In 1838, Justus von Liebig proposed that an acid is a hydrogen-containing compound whose hydrogen can be replaced by a metal. This redefinition was based on his extensive work on the chemical composition of organic acids, finishing the doctrinal shift from oxygen-based acids to hydrogen-based acids started by Davy. Liebig's definition, while completely empirical, remained in use for almost 50 years until the adoption of the Arrhenius definition.Arrhenius definition
 The first modern definition of acids and bases in molecular terms was devised by
The first modern definition of acids and bases in molecular terms was devised by Svante Arrhenius
Svante August Arrhenius ( , ; 19 February 1859 – 2 October 1927) was a Swedish scientist. Originally a physicist, but often referred to as a chemist, Arrhenius was one of the founders of the science of physical chemistry. He received the Nob ...
.Miessler G.L. and Tarr D.A. ''Inorganic Chemistry'' (2nd ed., Prentice-Hall 1999) p. 154 A hydrogen theory of acids, it followed from his 1884 work with Friedrich Wilhelm Ostwald
Friedrich Wilhelm Ostwald (; 4 April 1932) was a Baltic German chemist and philosopher. Ostwald is credited with being one of the founders of the field of physical chemistry, with Jacobus Henricus van 't Hoff, Walther Nernst, and Svante Arrhe ...
in establishing the presence of ions in aqueous solution and led to Arrhenius receiving the Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
in 1903.
As defined by Arrhenius:
*''an Arrhenius acid'' is a substance that dissociates in water to form hydrogen ions (H+); that is, an acid increases the concentration of H+ ions in an aqueous solution.
This causes the protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid ...
of water, or the creation of the hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid i ...
(H3O+) ion. Thus, in modern times, the symbol H+ is interpreted as a shorthand for H3O+, because it is now known that a bare proton does not exist as a free species in aqueous solution. This is the species which is measured by pH indicator
A pH indicator is a halochromic chemical compound added in small amounts to a solution so the pH (acidity or basicity) of the solution can be determined visually or spectroscopically by changes in absorption and/or emission properties. Hence, ...
s to measure the acidity or basicity of a solution.
*''an Arrhenius base is a substance that dissociates in water to form hydroxide (OH−) ions; that is, a base increases the concentration of OH− ions in an aqueous solution."
The Arrhenius definitions of acidity
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a ...
and alkalinity
Alkalinity (from ar, القلوي, al-qaly, lit=ashes of the saltwort) is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale.
Alkalinity is the strength ...
are restricted to aqueous solutions and are not valid for most non-aqueous solutions, and refer to the concentration of the solvent ions. Under this definition, pure H2SO4 and HCl dissolved in toluene are not acidic, and molten NaOH and solutions of calcium amide in liquid ammonia are not alkaline. This led to the development of the Brønsted-Lowry theory and subsequent Lewis theory to account for these non-aqueous
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be r ...
exceptions.
The reaction of an acid with a base is called a neutralization reaction. The products of this reaction are a salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quant ...
and water.
:acid + base → salt + water
In this traditional representation an acid–base neutralization reaction is formulated as a double-replacement reaction. For example, the reaction of hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
, HCl, with sodium hydroxide, NaOH, solutions produces a solution of sodium chloride, NaCl, and some additional water molecules.
:HCl(aq) + NaOH(aq) → NaCl(aq) + H2O
The modifier ( aq) in this equation was implied by Arrhenius, rather than included explicitly. It indicates that the substances are dissolved in water. Though all three substances, HCl, NaOH and NaCl are capable of existing as pure compounds, in aqueous solutions they are fully dissociated into the aquated ions H+, Cl−, Na+ and OH−.
Example: Baking Powder
When combined with water, the sodium bicarbonate and acid salts react to produce gaseouscarbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
.
Whether commercially or domestically prepared, the principles behind baking powder formulations remain the same. The acid-base reaction can be generically represented as shown:
:NaHCO3 + H+ → Na+ + CO2 + H2O
The real reactions are more complicated because the acids are complicated. For example, starting with baking soda and monocalcium phosphate, the reaction produces carbon dioxide by the following stoichiometry:John Brodie, John Godber "Bakery Processes, Chemical Leavening Agents" in Kirk-Othmer Encyclopedia of Chemical Technology 2001, John Wiley & Sons.
:14 NaHCO3 + 5 Ca(H2PO4)2 → 14 CO2 + Ca5(PO4)3OH + 7 Na2HPO4 + 13 H2O
A typical formulation (by weight) could call for 30% sodium bicarbonate, 5–12% monocalcium phosphate
Monocalcium phosphate is an inorganic compound with the chemical formula Ca(H2PO4)2 ("AMCP" or "CMP-A" for anhydrous monocalcium phosphate). It is commonly found as the monohydrate ("MCP" or "MCP-M"), Ca(H2PO4)2·H2O. Both salts are colourless ...
, and 21–26% sodium aluminium sulfate.
Alternately, a commercial baking powder might use sodium acid pyrophosphate
Disodium pyrophosphate or sodium acid pyrophosphate (SAPP) is an inorganic compound consisting of sodium cations and pyrophosphate anion. It is a white, water-soluble solid that serves as a buffering and chelating agent, with many applications ...
as one of the two acidic components instead of sodium aluminium sulfate.
Another typical acid in such formulations is cream of tartar
Potassium bitartrate, also known as potassium hydrogen tartrate, with formula K C4 H5 O6, is a byproduct of winemaking. In cooking, it is known as cream of tartar. It is processed from the potassium acid salt of tartaric acid (a carboxylic ac ...
( K C4 H5 O6), a derivative of tartaric acid
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally ...
.
Brønsted–Lowry definition
The Brønsted–Lowry definition, formulated in 1923, independently by Johannes Nicolaus Brønsted in Denmark and Martin Lowry in England, is based upon the idea ofprotonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid ...
of bases through the deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju. ...
of acids – that is, the ability of acids to "donate" hydrogen ions (H+)—otherwise known as protons—to bases, which "accept" them. – According to this page, the original definition was that "acids have a tendency to lose a proton""Removal and addition of a proton from the nucleus of an atom does not occur – it would require very much more energy than is involved in the dissociation of acids."
An acid–base reaction is, thus, the removal of a hydrogen ion from the acid and its addition to the base. The removal of a hydrogen ion from an acid produces its ''conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
'', which is the acid with a hydrogen ion removed. The reception of a proton by a base produces its ''conjugate acid
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
'', which is the base with a hydrogen ion added.
Unlike the previous definitions, the Brønsted–Lowry definition does not refer to the formation of salt and solvent, but instead to the formation of ''conjugate acids'' and ''conjugate bases'', produced by the transfer of a proton from the acid to the base. In this approach, acids and bases are fundamentally different in behavior from salts, which are seen as electrolytes, subject to the theories of Debye
The debye (symbol: D) (; ) is a CGS unit (a non- SI metric unit) of electric dipole momentTwo equal and opposite charges separated by some distance constitute an electric dipole. This dipole possesses an electric dipole moment whose value is g ...
, Onsager, and others. An acid and a base react not to produce a salt and a solvent, but to form a new acid and a new base. The concept of neutralization is thus absent. Brønsted–Lowry acid–base behavior is formally independent of any solvent, making it more all-encompassing than the Arrhenius model. The calculation of pH under the Arrhenius model depended on alkalis (bases) dissolving in water ( aqueous solution). The Brønsted–Lowry model expanded what could be pH tested using insoluble and soluble solutions (gas, liquid, solid).
The general formula for acid–base reactions according to the Brønsted–Lowry definition is:
:HA + B → BH+ + A−
where HA represents the acid, B represents the base, BH+ represents the conjugate acid of B, and A− represents the conjugate base of HA.
For example, a Brønsted–Lowry model for the dissociation of hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
(HCl) in aqueous solution would be the following:
:HCl + H2O H3O+ + Cl−
The removal of H+ from the HCl produces the chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride sa ...
ion, Cl−, the conjugate base of the acid. The addition of H+ to the H2O (acting as a base) forms the hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid i ...
ion, H3O+, the conjugate acid of the base.
Water is amphoteric
In chemistry, an amphoteric compound () is a molecule or ion that can react both as an acid and as a base. What exactly this can mean depends on which definitions of acids and bases are being used.
One type of amphoteric species are amphipro ...
—that is, it can act as both an acid and a base. The Brønsted–Lowry model explains this, showing the dissociation of water into low concentrations of hydronium and hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
ions:
:H2O + H2O H3O+ + OH−
This equation is demonstrated in the image below:
 Here, one molecule of water acts as an acid, donating an H+ and forming the conjugate base, OH−, and a second molecule of water acts as a base, accepting the H+ ion and forming the conjugate acid, H3O+.
As an example of water acting as an acid, consider an aqueous solution of
Here, one molecule of water acts as an acid, donating an H+ and forming the conjugate base, OH−, and a second molecule of water acts as a base, accepting the H+ ion and forming the conjugate acid, H3O+.
As an example of water acting as an acid, consider an aqueous solution of pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a ...
, C5H5N.
:C5H5N + H2O 5H5NHsup>+ + OH−
In this example, a water molecule is split into a hydrogen ion, which is donated to a pyridine molecule, and a hydroxide ion.
In the Brønsted–Lowry model, the solvent does not necessarily have to be water, as is required by the Arrhenius Acid-Base model. For example, consider what happens when acetic acid, CH3COOH, dissolves in liquid ammonia.
: + +
An H+ ion is removed from acetic acid, forming its conjugate base, the acetate ion, CH3COO−. The addition of an H+ ion to an ammonia molecule of the solvent creates its conjugate acid, the ammonium ion, .
The Brønsted–Lowry model calls hydrogen-containing substances (like HCl) acids. Thus, some substances, which many chemists considered to be acids, such as SO3 or BCl3, are excluded from this classification due to lack of hydrogen. Gilbert N. Lewis wrote in 1938, "To restrict the group of acids to those substances that contain hydrogen interferes as seriously with the systematic understanding of chemistry as would the restriction of the term oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxi ...
to substances containing oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
." Furthermore, KOH and KNH2 are not considered Brønsted bases, but rather salts containing the bases OH− and .
Lewis definition
The hydrogen requirement of Arrhenius and Brønsted–Lowry was removed by the Lewis definition of acid–base reactions, devised by Gilbert N. Lewis in 1923, – Table of discoveries attributes the date of publication/release for the Lewis theory as 1924. in the same year as Brønsted–Lowry, but it was not elaborated by him until 1938. Instead of defining acid–base reactions in terms of protons or other bonded substances, the Lewis definition defines a base (referred to as a ''Lewis base'') to be a compound that can donate an '' electron pair'', and an acid (a ''Lewis acid'') to be a compound that can receive this electron pair. For example, boron trifluoride, BF3 is a typical Lewis acid. It can accept a pair of electrons as it has a vacancy in itsoctet
Octet may refer to:
Music
* Octet (music), ensemble consisting of eight instruments or voices, or composition written for such an ensemble
** String octet, a piece of music written for eight string instruments
*** Octet (Mendelssohn), 1825 compos ...
. The fluoride ion has a full octet and can donate a pair of electrons. Thus
:BF3 + F− →
is a typical Lewis acid, Lewis base reaction. All compounds of group 13 elements with a formula AX3 can behave as Lewis acids. Similarly, compounds of group 15
A pnictogen ( or ; from grc, πνῑ́γω "to choke" and -gen, "generator") is any of the chemical elements in group 15 of the periodic table. Group 15 is also known as the nitrogen group or nitrogen family. Group 15 consists of the ...
elements with a formula DY3, such as amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
s, NR3, and phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
s, PR3, can behave as Lewis bases. Adducts between them have the formula X3A←DY3 with a dative covalent bond, shown symbolically as ←, between the atoms A (acceptor) and D (donor). Compounds of group 16 with a formula DX2 may also act as Lewis bases; in this way, a compound like an ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be ...
, R2O, or a thioether
In organic chemistry, an organic sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A su ...
, R2S, can act as a Lewis base. The Lewis definition is not limited to these examples. For instance, carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
acts as a Lewis base when it forms an adduct with boron trifluoride, of formula F3B←CO.
Adducts involving metal ions are referred to as co-ordination compounds; each ligand donates a pair of electrons to the metal ion. The reaction
: g(H2O)4sup>+ + 2NH3 → g(NH3)2sup>+ + 4H2O
can be seen as an acid–base reaction in which a stronger base (ammonia) replaces a weaker one (water)
The Lewis and Brønsted–Lowry definitions are consistent with each other since the reaction
:H+ + OH− H2O
is an acid–base reaction in both theories.
Solvent system definition
One of the limitations of the Arrhenius definition is its reliance on water solutions.Edward Curtis Franklin
Edward Curtis Franklin (March 1, 1862 – February 13, 1937) was an American chemist.
Biography
Edward Franklin was born in Geary County, Kansas. He entered the University of Kansas at the age of 22, obtaining his major in chemistry in 1888. ...
studied the acid–base reactions in liquid ammonia in 1905 and pointed out the similarities to the water-based Arrhenius theory. Albert F.O. Germann, working with liquid phosgene, , formulated the solvent-based theory in 1925, thereby generalizing the Arrhenius definition to cover aprotic solvents.
Germann pointed out that in many solutions, there are ions in equilibrium with the neutral solvent molecules:
*solvonium ions: a generic name for positive ions. (The term ''solvonium'' has replaced the older term '' lyonium ions'': positive ions formed by protonation of solvent molecules.)
*solvate ions: a generic name for negative ions. (The term ''solvate'' has replaced the older term '' lyate ions'': negative ions formed by deprotonation of solvent molecules.)
For example, water and ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
undergo such dissociation into hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid i ...
and hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
, and ammonium and amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it i ...
, respectively:
:2 +
:2 +
Some aprotic systems also undergo such dissociation, such as dinitrogen tetroxide into nitrosonium and nitrate, antimony trichloride
Antimony trichloride is the chemical compound with the formula SbCl3. It is a soft colorless solid with a pungent odor and was known to alchemists as butter of antimony.
Preparation
Antimony trichloride is prepared by reaction of chlorine with an ...
into dichloroantimonium and tetrachloroantimonate, and phosgene into chlorocarboxonium and chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride sa ...
:
: +
:2 +
: +
A solute that causes an increase in the concentration of the solvonium ions and a decrease in the concentration of solvate ions is defined as an ''acid''. A solute that causes an increase in the concentration of the solvate ions and a decrease in the concentration of the solvonium ions is defined as a ''base''.
Thus, in liquid ammonia, (supplying ) is a strong base, and (supplying ) is a strong acid. In liquid sulfur dioxide (), thionyl compounds (supplying ) behave as acids, and sulfites (supplying ) behave as bases.
The non-aqueous acid–base reactions in liquid ammonia are similar to the reactions in water:
: + →
: + →
Nitric acid can be a base in liquid sulfuric acid:
: + 2 → + + 2
The unique strength of this definition shows in describing the reactions in aprotic solvents; for example, in liquid :
: + → +
Because the solvent system definition depends on the solute as well as on the solvent itself, a particular solute can be either an acid or a base depending on the choice of the solvent: is a strong acid in water, a weak acid in acetic acid, and a weak base in fluorosulfonic acid; this characteristic of the theory has been seen as both a strength and a weakness, because some substances (such as and ) have been seen to be acidic or basic on their own right. On the other hand, solvent system theory has been criticized as being too general to be useful. Also, it has been thought that there is something intrinsically acidic about hydrogen compounds, a property not shared by non-hydrogenic solvonium salts.
Lux–Flood definition
This acid–base theory was a revival of the oxygen theory of acids and bases proposed by German chemist Hermann Lux in 1939, further improved by Håkon Flood circa 1947 and is still used in moderngeochemistry
Geochemistry is the science that uses the tools and principles of chemistry to explain the mechanisms behind major geological systems such as the Earth's crust and its oceans. The realm of geochemistry extends beyond the Earth, encompassing the ...
and electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
of molten salt
Molten salt is salt which is solid at standard temperature and pressure but enters the liquid phase due to elevated temperature. Regular table salt has a melting point of 801 °C (1474°F) and a heat of fusion of 520 J/g.Journal of Chemical T ...
s. This definition describes an acid as an oxide ion () acceptor and a base as an oxide ion donor. For example:
: + →
: + →
: + → + 2
This theory is also useful in the systematisation of the reactions of noble gas compound
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularl ...
s, especially the xenon oxides, fluorides, and oxofluorides.
Usanovich definition
Mikhail Usanovich developed a general theory that does not restrict acidity to hydrogen-containing compounds, but his approach, published in 1938, was even more general than Lewis theory. Usanovich's theory can be summarized as defining an acid as anything that accepts negative species or donates positive ones, and a base as the reverse. This defined the concept ofredox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
(oxidation-reduction) as a special case of acid–base reactions
Some examples of Usanovich acid–base reactions include:
: + → 2 + (species exchanged: anion)
: + → 6 + 2 (species exchanged: 3 anions)
: + → 2 + 2 (species exchanged: 2 electrons)
Rationalizing the strength of Lewis acid–base interactions
HSAB theory
In 1963,Ralph Pearson
Ralph Gottfrid Pearson (January 12, 1919 – October 12, 2022) was an American physical inorganic chemist best known for the development of the concept of hard and soft acids and bases (HSAB).
He received his Ph.D. in physical chemistry in 1943 ...
proposed a qualitative concept known as the Hard and Soft Acids and Bases principle. later made quantitative with help of Robert Parr in 1984. 'Hard' applies to species that are small, have high charge states, and are weakly polarizable. 'Soft' applies to species that are large, have low charge states and are strongly polarizable. Acids and bases interact, and the most stable interactions are hard–hard and soft–soft. This theory has found use in organic and inorganic chemistry.
ECW model
The ECW model created by Russell S. Drago is a quantitative model that describes and predicts the strength of Lewis acid base interactions, −Δ''H''. The model assigned ''E'' and ''C'' parameters to many Lewis acids and bases. Each acid is characterized by an ''E''A and a ''C''A. Each base is likewise characterized by its own ''E''B and ''C''B. The ''E'' and ''C'' parameters refer, respectively, to the electrostatic and covalent contributions to the strength of the bonds that the acid and base will form. The equation is : −Δ''H'' = ''E''A''E''B + ''C''A''C''B + ''W'' The ''W'' term represents a constant energy contribution for acid–base reaction such as the cleavage of a dimeric acid or base. The equation predicts reversal of acids and base strengths. The graphical presentations of the equation show that there is no single order of Lewis base strengths or Lewis acid strengths.Acid–base equilibrium
The reaction of a strong acid with a strong base is essentially a quantitative reaction. For example, :HCl(aq) + Na(OH)(aq) → H2O + NaCl(aq) In this reaction both the sodium and chloride ions are spectators as the neutralization reaction, :H+ + OH− → H2O does not involve them. With weak bases addition of acid is not quantitative because a solution of a weak base is abuffer solution
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is ...
. A solution of a weak acid is also a buffer solution. When a weak acid reacts with a weak base an equilibrium mixture is produced. For example, adenine
Adenine () ( symbol A or Ade) is a nucleobase (a purine derivative). It is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The three others are guanine, cytosine and thymine. Its deri ...
, written as AH, can react with a hydrogen phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
ion, .
:AH + A− +
The equilibrium constant for this reaction can be derived from the acid dissociation constants of adenine and of the dihydrogen phosphate ion.
: − += ''K''a1 H:[] += ''K''a2[]
The notation [X] signifies "concentration of X". When these two equations are combined by eliminating the hydrogen ion concentration, an expression for the equilibrium constant, ''K'' is obtained.
: −[] = ''K''[AH] []; ''K'' =
Acid–alkali reaction
An acid–alkali reaction is a special case of an acid–base reaction, where the base used is also an alkali. When an acid reacts with an alkali salt (a metal hydroxide), the product is a metalsalt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quant ...
and water. Acid–alkali reactions are also neutralization reactions.
In general, acid–alkali reactions can be simplified to
: (aq) + (aq) →
by omitting spectator ions.
Acids are in general pure substances that contain hydrogen cations () or cause them to be produced in solutions. Hydrochloric acid () and sulfuric acid () are common examples. In water, these break apart into ions:
: → (aq) + (aq)
: → (aq) + (aq)
The alkali breaks apart in water, yielding dissolved hydroxide ions:
: → (aq) + (aq)
See also
*Acid–base titration
An acid–base titration is a method of quantitative analysis for determining the concentration of an acid or base by exactly neutralizing it with a standard solution of base or acid having known concentration. A pH indicator is used to monit ...
*Deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju. ...
*Donor number In chemistry a donor number (DN) is a quantitative measure of Lewis basicity. A donor number is defined as the negative enthalpy value for the 1:1 adduct formation between a Lewis base and the standard Lewis acid SbCl5 (antimony pentachloride), in ...
* Electron configuration
*Gutmann–Beckett method
In chemistry, the Gutmann–Beckett method is an experimental procedure used by chemists to assess the Lewis acidity of molecular species. Triethylphosphine oxide (, TEPO) is used as a probe molecule and systems are evaluated by 31P-NMR spectr ...
*Lewis structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons t ...
*Nucleophilic substitution
In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The ...
*Neutralization (chemistry)
In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react quantitatively with each other. In a reaction in water, neutralization results in there being no excess of hydrogen o ...
*Protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid ...
*Redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
reactions
* Resonance (chemistry)
Notes
References
Sources
* * * *External links
Acid-base Physiology: an on-line text
{{DEFAULTSORT:Acid-Base Reaction Acids Bases (chemistry) Acid-base chemistry Equilibrium chemistry