Auwers Synthesis on:
[Wikipedia]
[Google]
[Amazon]
The Auwers synthesis is a series of  The first step in this procedure is an acid catalyzed
The first step in this procedure is an acid catalyzed
organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical rea ...
s forming a flavonol
Flavonols are a class of flavonoids that have the 3-hydroxyflavone backbone (IUPAC name : 3-hydroxy-2-phenylchromen-4-one). Their diversity stems from the different positions of the phenolic -OH groups. They are distinct from flavanols (with " ...
from a coumarone
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen ...
. This reaction was first reported by Karl von Auwers
Karl Friedrich von Auwers (September 16, 1863 – May 3, 1939) was a German chemist, and was the academic adviser of both Karl Ziegler and Georg Wittig at the University of Marburg.
Life
Karl Friedrich von Auwers was born the son of the renowned ...
in 1908.K. v. Auwers, E. Auffenberg, "Über Cumaranone und Hydrindone", ''Ber. Dtsch. Chem. Ges.
''Chemische Berichte'' (usually abbreviated as ''Ber.'' or ''Chem. Ber.'') was a German-language scientific journal of all disciplines of chemistry founded in 1868. It was one of the oldest scientific journals in chemistry, until it merged with ' ...
'', 52, 92-113 (1919) ().
aldol condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration ...
between benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-like odor. ...
and a 3-cyclooxapentanone to an o-hydroxychalcone. Bromination
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polyme ...
of the alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
group gives a dibromo-adduct which rearranges to the flavonol by reaction with potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which expl ...
.
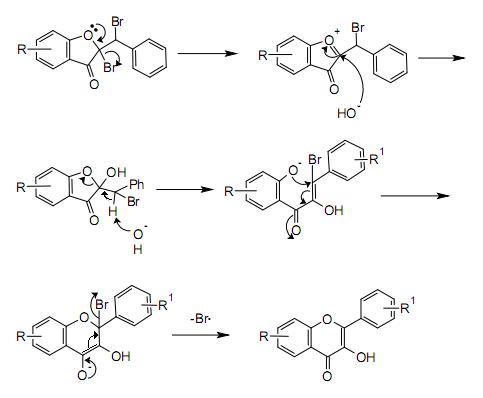
Mechanism
A possible mechanism for the rearrangement step is shown below:
See also
* Algar–Flynn–Oyamada reaction * Allan–Robinson reactionReferences
{{Organic reactions Heterocycle forming reactions Name reactions Oxygen heterocycle forming reactions Ring expansion reactions Carbon-carbon bond forming reactions