Arrow pushing on:
[Wikipedia]
[Google]
[Amazon]
Arrow pushing or electron pushing is a technique used to describe the progression of
 The representation of reaction mechanisms using curved arrows to indicate electron flow was developed by Sir Robert Robinson in 1922. Organic chemists use two types of arrows within molecular structures to describe electron movements. Single electron's trajectories are designated with single barbed arrows, whereas double-barbed arrows show movement of electron pairs. The arrow's tail is drawn at either a lone pair of electrons on an atom or a bond between atoms, an electron source or area where there is relatively high electron density. Its head points towards electron sinks, or areas of relatively low electron density.
The representation of reaction mechanisms using curved arrows to indicate electron flow was developed by Sir Robert Robinson in 1922. Organic chemists use two types of arrows within molecular structures to describe electron movements. Single electron's trajectories are designated with single barbed arrows, whereas double-barbed arrows show movement of electron pairs. The arrow's tail is drawn at either a lone pair of electrons on an atom or a bond between atoms, an electron source or area where there is relatively high electron density. Its head points towards electron sinks, or areas of relatively low electron density.

 When a bond is broken, electrons leave where the bond was; this is represented by a curved arrow pointing away from the bond and ending with the arrow pointing towards the next unoccupied molecular orbital. The electrons can be transferred to a specific atom or can be transferred to a single (sigma) bond, thus making it a double (pi) bond, but the arrow is always pointing towards a specific atom, because electrons always move to a new atom whenever they are "pushed". Organic chemists represent the formation of a bond by a curved arrow pointing between two species.
For clarity, when pushing arrows, it is best to draw the arrows starting from a lone pair of electrons or a σ or π bond and ending in a position that can accept a pair of electrons, allowing the reader to know exactly which electrons are moving and where they are ending. Bonds are broken in places where a corresponding antibonding orbital is filled. Some authorities allow the simplification that an arrow can originate at a formal negative charge that corresponds to a lone pair. However, not all formal negative charges correspond to the presence of a lone pair (e.g., the B in F4B−), and care needs to be taken with this usage.
When a bond is broken, electrons leave where the bond was; this is represented by a curved arrow pointing away from the bond and ending with the arrow pointing towards the next unoccupied molecular orbital. The electrons can be transferred to a specific atom or can be transferred to a single (sigma) bond, thus making it a double (pi) bond, but the arrow is always pointing towards a specific atom, because electrons always move to a new atom whenever they are "pushed". Organic chemists represent the formation of a bond by a curved arrow pointing between two species.
For clarity, when pushing arrows, it is best to draw the arrows starting from a lone pair of electrons or a σ or π bond and ending in a position that can accept a pair of electrons, allowing the reader to know exactly which electrons are moving and where they are ending. Bonds are broken in places where a corresponding antibonding orbital is filled. Some authorities allow the simplification that an arrow can originate at a formal negative charge that corresponds to a lone pair. However, not all formal negative charges correspond to the presence of a lone pair (e.g., the B in F4B−), and care needs to be taken with this usage.
 For example,
For example, 
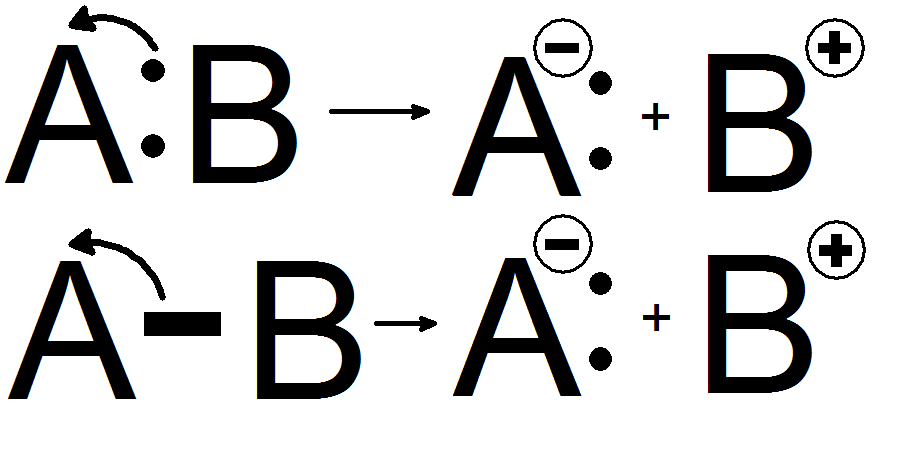
 In the first stage of this reaction (solvolysis), the C-L bond breaks and both electrons from that bond join L (the
In the first stage of this reaction (solvolysis), the C-L bond breaks and both electrons from that bond join L (the
 Because an SN2 reaction proceeds with the substitution of a leaving group with a nucleophile, the SN designation is used. Because this mechanism proceeds with the interaction of two species at the transition state, it is referred to as a bimolecular process, resulting in the SN2 designation. An SN2 reaction is a concerted process, which means that the bonds are breaking and forming concurrently. Thus, the electron movement shown by the arrow pushing is happening simultaneously. An SN2 reaction has one step.
Because an SN2 reaction proceeds with the substitution of a leaving group with a nucleophile, the SN designation is used. Because this mechanism proceeds with the interaction of two species at the transition state, it is referred to as a bimolecular process, resulting in the SN2 designation. An SN2 reaction is a concerted process, which means that the bonds are breaking and forming concurrently. Thus, the electron movement shown by the arrow pushing is happening simultaneously. An SN2 reaction has one step.
 Because initial formation of a
Because initial formation of a  E1 eliminations proceed with the Elimination of a leaving group leading to the E designation. Because this mechanism proceeds with the initial dissociation of a single starting material forming a carbocation, this process is considered a uni-molecular reaction. The involvement of only 1 species in the initial phase of the reaction enhances the mechanistic designation to E1.
E1 eliminations proceed with the Elimination of a leaving group leading to the E designation. Because this mechanism proceeds with the initial dissociation of a single starting material forming a carbocation, this process is considered a uni-molecular reaction. The involvement of only 1 species in the initial phase of the reaction enhances the mechanistic designation to E1.
 Similar to the relationship between E1 eliminations and SN1 mechanisms, E2 eliminations often occur in competition with SN2 reactions. This observation is most often noted when the base is also a nucleophile. In order to minimize this competition, non-nucleophilic bases are commonly used to effect E2 eliminations.
Similar to the relationship between E1 eliminations and SN1 mechanisms, E2 eliminations often occur in competition with SN2 reactions. This observation is most often noted when the base is also a nucleophile. In order to minimize this competition, non-nucleophilic bases are commonly used to effect E2 eliminations.
 E2 eliminations proceed through initial extraction of a proton by a base or nucleophile leading to Elimination of a leaving group justifying the E designation. Because this mechanism proceeds through the interaction of two species (substrate and base/nucleophile), E2 reactions are recognized as bi-molecular. Thus, the involvement of 2 species in the initial phase of the reaction enhances the mechanistic designation to E2.
E2 eliminations proceed through initial extraction of a proton by a base or nucleophile leading to Elimination of a leaving group justifying the E designation. Because this mechanism proceeds through the interaction of two species (substrate and base/nucleophile), E2 reactions are recognized as bi-molecular. Thus, the involvement of 2 species in the initial phase of the reaction enhances the mechanistic designation to E2.



Arrow-Pushing in Organic Chemistry: An Easy Approach to Understanding Reaction Mechanisms - Second Edition
(John Wiley & Sons, 2017) *Daniel P. Weeks, Pushing Electrons: A Guide for Students of Organic Chemistry, (Brooks Cole, 1998) *Abhik Ghosh, Steffen Berg
(John Wiley & Sons, 2014) *Robert B. Grossman, The Art of Writing Reasonable Organic Reaction Mechanisms, (Springer, 2007)
MIT.edu
OpenCourseWare: Organic Chemistry I
HaverFord.edu
Organic Chemistry Lectures, Videos and Text
Virtual Textbook of Organic Chemistry {{DEFAULTSORT:Arrow Pushing Organic chemistry Chemical reactions Reaction mechanisms
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
reaction
Reaction may refer to a process or to a response to an action, event, or exposure:
Physics and chemistry
*Chemical reaction
*Nuclear reaction
*Reaction (physics), as defined by Newton's third law
*Chain reaction (disambiguation).
Biology and me ...
mechanisms. It was first developed by Sir Robert Robinson. In using arrow pushing, "curved arrows" or "curly arrows" are drawn on the structural formulae of reactants in a chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between ...
to show the reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
. The arrows illustrate the movement of electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s as bonds between atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s are broken and formed. It is important to note that arrow pushing never directly show the movement of atoms; it is used to show the movement of electron density, which indirectly shows the movement of atoms themselves. Arrow pushing is also used to describe how positive and negative charges
Charge or charged may refer to:
Arts, entertainment, and media Films
* ''Charge, Zero Emissions/Maximum Speed'', a 2011 documentary
Music
* ''Charge'' (David Ford album)
* ''Charge'' (Machel Montano album)
* '' Charge!!'', an album by The Aqu ...
are distributed around organic molecule
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The s ...
s through resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillatin ...
. It is important to remember, however, that arrow pushing is a formalism and electrons (or rather, electron density) do not move around so neatly and discretely in reality.
Arrow pushing has been extended to inorganic chemistry
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disci ...
, especially to the chemistry of s- and p-block
Block or blocked may refer to:
Arts, entertainment and media Broadcasting
* Block programming, the result of a programming strategy in broadcasting
* W242BX, a radio station licensed to Greenville, South Carolina, United States known as ''96.3 ...
elements. It has been shown to work well for hypervalent
In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded Octet rule, octet) is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. P ...
compounds.
Notation
Breaking of bonds
Acovalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
joining atoms in an organic molecule consists of a group of two electrons. Such a group is referred to as an electron pair. Reactions in organic chemistry proceed through the sequential breaking and formation of such bonds. Organic chemists recognize two processes for the breaking of a chemical bond. These processes are known as homolytic cleavage and heterolytic cleavage.
Homolytic bond cleavage
Homolyticbond cleavage
In chemistry, bond cleavage, or bond fission, is the splitting of chemical bonds. This can be generally referred to as dissociation when a molecule is cleaved into two or more fragments.
In general, there are two classifications for bond cleavag ...
is a process where the electron pair comprising a bond is split, causing the bond to break. This is denoted by two single barbed curved arrows pointing away from the bond. The consequence of this process is the retention of a single unpaired electron denoted by a dot on each of the atoms that were formerly joined by a bond. The single electron movement can be denoted by a curved arrow commonly referred to as a fish hook. These single electron species are known as free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
s. Heat or light are required to provide enough energy for this process to occur.
:  For example,
For example, Ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nanometer, nm (with a corresponding frequency around 30 Hertz, PHz) to 400 nm (750 Hertz, THz), shorter than that of visible light, but longer than ...
light causes the chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
-chlorine bond to break homolytically. The pair of electrons become split, denoted by the two fish hook arrows between both atoms pointing to both chlorine atoms. After the reaction occurs, it leads to both chlorine molecules left with a single unpaired electron. This is the initiation stage of free radical halogenation
In organic chemistry, free-radical halogenation is a type of halogenation. This chemical reaction is typical of alkanes and alkyl-substituted aromatics under application of UV light. The reaction is used for the industrial synthesis of chloroform ( ...
.
: Heterolytic bond cleavage
Heterolytic bond cleavage is a process where the electron pair that comprised a bond moves to one of the atoms that was formerly joined by a bond. The bond breaks, forming a negatively chargedspecies
In biology, a species is the basic unit of classification and a taxonomic rank of an organism, as well as a unit of biodiversity. A species is often defined as the largest group of organisms in which any two individuals of the appropriate s ...
(an anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
) and a positively charged species (a cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
). The anion is the species that retains the electrons from the bond while the cation is stripped of the electrons from the bond. The anion usually forms on the most electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
atom, in this example atom A. This is because the most electronegative atom will naturally attract electrons towards itself more strongly, leading to its negative charge.
: 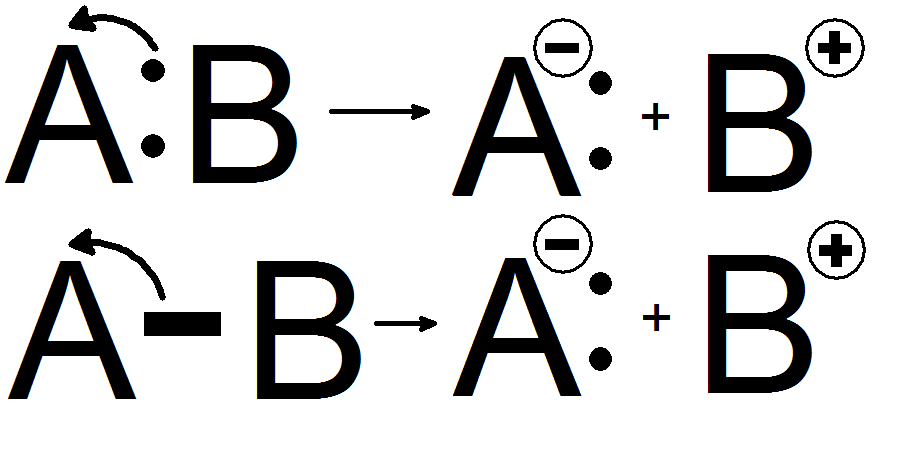
Acid-base reactions
A Lewis acid-base reaction occurs when a molecule with a lone electron pair, or a base, donates its electrons to an electron-pair acceptor, also known as an acid. This can be shown in a reaction with a curved arrow pointing from the nonbonding electron pair to the electron acceptor. This can be shown in a reaction with a curved arrow pointing from the nonbonding electron pair to the electron acceptor. In a reaction involving Brønsted-Lowry acids and bases, the arrows are used in the same manner, and they help to indicate the attacking proton. In a Brønsted-Lowry acid-base reaction the arrow will begin from the acid, the proton acceptor, to the base, the proton donor.SN1 reactions
An SN1 reaction occurs when a molecule separates into a positively charged component and a negatively charged component. This generally occurs in highly polarsolvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s through a process called solvolysis
In chemistry, solvolysis is a type of nucleophilic substitution (S1/S2) or elimination reaction, elimination where the nucleophile is a solvent molecule. Characteristic of S1 reactions, solvolysis of a chirality (chemistry), chiral reactant affor ...
. The positively charged component then reacts with a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
forming a new compound. SN1 reactions are reactions whose rate is dependent only on haloalkane concentration.
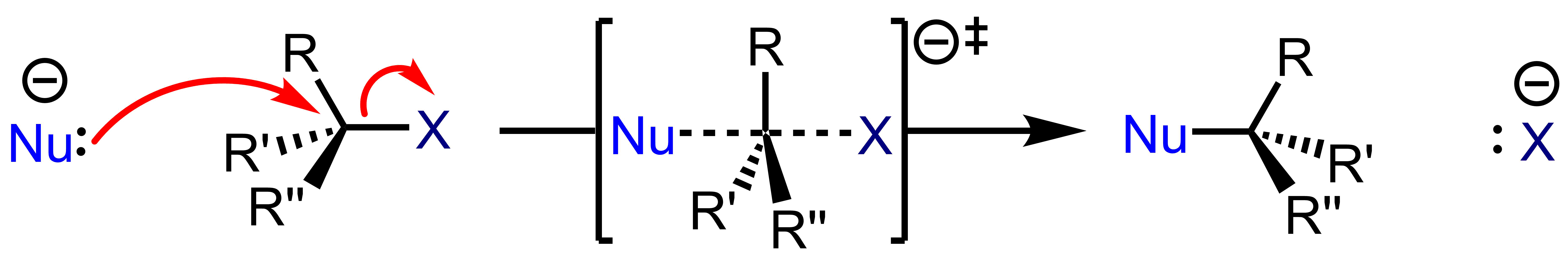
 In the first stage of this reaction (solvolysis), the C-L bond breaks and both electrons from that bond join L (the
In the first stage of this reaction (solvolysis), the C-L bond breaks and both electrons from that bond join L (the leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited t ...
) to form L− and R3C+ ions. This is represented by the curved arrow pointing away from the C-L bond and towards L. The nucleophile Nu−, being attracted to the R3C+, then donates a pair of electrons forming a new C-Nu bond.
Because an SN1 reaction proceeds with the Substitution of a leaving group with a Nucleophile, the SN designation is used. Because the initial solvolysis step in this reaction involves a single molecule dissociating from its leaving group, the initial stage of this process is considered a uni-molecular reaction. The involvement of only 1 species in the initial phase of the reaction enhances the mechanistic designation to SN1. An SN1 reaction has two steps.
SN2 reactions
An SN2 reaction occurs when a nucleophile displaces a leaving group residing on a molecule from the backside of the leaving group. This displacement or substitution results in the formation of a substitution product with inversion of stereochemical configuration. The nucleophile forms a bond with its lone pair as the electron source. The electron sink which ultimately accepts the electron density is the nucleofuge (leaving group), with bond forming and bond breaking occurring simultaneously at the transition state (marked with a double-dagger). The rates of SN2 reactions are dependent on the concentration of the haloalkane and the nucleophile. Because an SN2 reaction proceeds with the substitution of a leaving group with a nucleophile, the SN designation is used. Because this mechanism proceeds with the interaction of two species at the transition state, it is referred to as a bimolecular process, resulting in the SN2 designation. An SN2 reaction is a concerted process, which means that the bonds are breaking and forming concurrently. Thus, the electron movement shown by the arrow pushing is happening simultaneously. An SN2 reaction has one step.
Because an SN2 reaction proceeds with the substitution of a leaving group with a nucleophile, the SN designation is used. Because this mechanism proceeds with the interaction of two species at the transition state, it is referred to as a bimolecular process, resulting in the SN2 designation. An SN2 reaction is a concerted process, which means that the bonds are breaking and forming concurrently. Thus, the electron movement shown by the arrow pushing is happening simultaneously. An SN2 reaction has one step.
E1 eliminations
An E1 elimination occurs when aproton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
adjacent to a positive charge leaves and generates a double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
.
cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
is necessary for E1 reactions to occur, E1 reactions are often observed as side reactions to SN1 mechanisms.
 E1 eliminations proceed with the Elimination of a leaving group leading to the E designation. Because this mechanism proceeds with the initial dissociation of a single starting material forming a carbocation, this process is considered a uni-molecular reaction. The involvement of only 1 species in the initial phase of the reaction enhances the mechanistic designation to E1.
E1 eliminations proceed with the Elimination of a leaving group leading to the E designation. Because this mechanism proceeds with the initial dissociation of a single starting material forming a carbocation, this process is considered a uni-molecular reaction. The involvement of only 1 species in the initial phase of the reaction enhances the mechanistic designation to E1.
E2 eliminations
An E2 elimination occurs when a proton adjacent to a leaving group is extracted by a base with simultaneous elimination of a leaving group and generation of a double bond.Addition reactions
Addition reaction
In organic chemistry, an addition reaction is, in simplest terms, an organic reaction where two or more molecules combine to form a larger one (the adduct)..
Addition reactions are limited to chemical compounds that have multiple bonds, such as ...
s occur when nucleophiles react with carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
s. When a nucleophile adds to a simple aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
or ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
, the result is a 1,2-addition. When a nucleophile adds to a conjugated carbonyl system, the result is a 1,4-addition. The designations 1,2 and 1,4 are derived from numbering the atoms of the starting compound where the oxygen is labeled “1” and each atom adjacent to the oxygen are sequentially numbered out to the site of nucleophilic addition. A 1,2-addition occurs with nucleophilic addition to position 2 while a 1,4-addition occurs with nucleophilic addition to position 4.
Addition-elimination reactions
Addition-elimination reactions are addition reactions immediately followed by elimination reactions. In general, these reactions take place whenester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
s (or related functional groups) react with nucleophiles. In fact, the only requirement for an addition-elimination reaction to proceed is that the group being eliminated is a better leaving group than the incoming nucleophile.
See also
*Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, Mechanistic Organ ...
* Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
* SN1 reaction
* SN2 reaction
* Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 ...
Notes
References
*Daniel E. LevyArrow-Pushing in Organic Chemistry: An Easy Approach to Understanding Reaction Mechanisms - Second Edition
(John Wiley & Sons, 2017) *Daniel P. Weeks, Pushing Electrons: A Guide for Students of Organic Chemistry, (Brooks Cole, 1998) *Abhik Ghosh, Steffen Berg
(John Wiley & Sons, 2014) *Robert B. Grossman, The Art of Writing Reasonable Organic Reaction Mechanisms, (Springer, 2007)
External links
MIT.edu
OpenCourseWare: Organic Chemistry I
HaverFord.edu
Organic Chemistry Lectures, Videos and Text
Virtual Textbook of Organic Chemistry {{DEFAULTSORT:Arrow Pushing Organic chemistry Chemical reactions Reaction mechanisms