Anode Rays on:
[Wikipedia]
[Google]
[Amazon]

 An anode ray (also positive ray or canal ray) is a beam of positive ions that is created by certain types of gas-discharge tubes. They were first observed in
An anode ray (also positive ray or canal ray) is a beam of positive ions that is created by certain types of gas-discharge tubes. They were first observed in
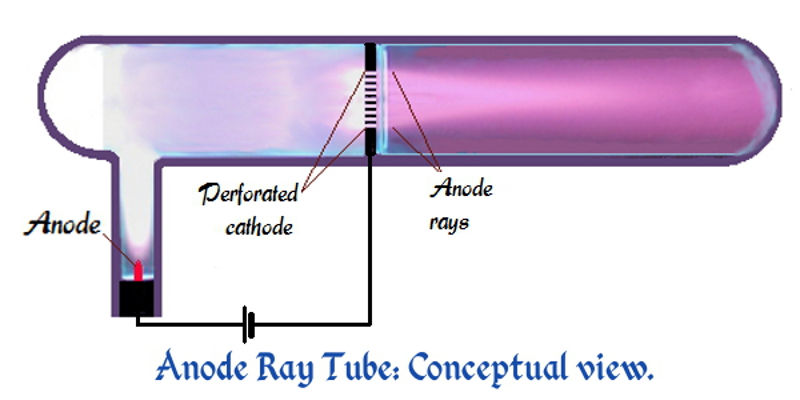
 Goldstein used a gas-discharge tube which had a perforated
Goldstein used a gas-discharge tube which had a perforated
Rays Of Positive Electricity by J.J. Thomson ''Proceedings of the Royal Society'', A 89, 1-20 (1913)
at The Cathode Ray Tube site German inventions Ionizing radiation Luminescence Mass spectrometry

 An anode ray (also positive ray or canal ray) is a beam of positive ions that is created by certain types of gas-discharge tubes. They were first observed in
An anode ray (also positive ray or canal ray) is a beam of positive ions that is created by certain types of gas-discharge tubes. They were first observed in Crookes tube
A Crookes tube (also Crookes–Hittorf tube) is an early experimental electrical discharge tube, with partial vacuum, invented by English physicist William Crookes and others around 1869-1875, in which cathode rays, streams of electrons, were ...
s during experiments by the German
German(s) may refer to:
* Germany (of or related to)
**Germania (historical use)
* Germans, citizens of Germany, people of German ancestry, or native speakers of the German language
** For citizens of Germany, see also German nationality law
**Ger ...
scientist
A scientist is a person who conducts scientific research to advance knowledge in an area of the natural sciences.
In classical antiquity, there was no real ancient analog of a modern scientist. Instead, philosophers engaged in the philosophica ...
Eugen Goldstein
Eugen Goldstein (; 5 September 1850 – 25 December 1930) was a German physicist. He was an early investigator of discharge tubes, the discoverer of anode rays or canal rays, later identified as positive ions in the gas phase including the hy ...
, in 1886. Later work on anode rays by Wilhelm Wien and J. J. Thomson led to the development of mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is u ...
.
Anode ray tube

 Goldstein used a gas-discharge tube which had a perforated
Goldstein used a gas-discharge tube which had a perforated cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction i ...
. When an electrical potential of several thousand volts is applied between the cathode and anode, faint luminous "rays" are seen extending from the holes in the back of the cathode. These rays are beams of particles moving in a direction opposite to the "cathode ray
Cathode rays or electron beam (e-beam) are streams of electrons observed in discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to el ...
s", which are streams of electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary partic ...
s which move toward the anode. Goldstein called these positive rays ''Kanalstrahlen'', "channel rays", or "canal rays", because these rays passed through the holes or ''channels'' in the cathode.
The process by which anode rays are formed in a gas-discharge anode ray tube is as follows. When the high voltage is applied to the tube, its electric field accelerates the small number of ions (electrically charged atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas ...
s) always present in the gas, created by natural processes such as radioactivity. These collide with atoms of the gas, knocking electrons off of them and creating more positive ions. These ions and electrons in turn strike more atoms, creating more positive ions in a chain reaction. The positive ions are all attracted to the negative cathode, and some pass through the holes in the cathode. These are the anode rays.
By the time they reach the cathode, the ions have been accelerated to a sufficient speed such that when they collide with other atoms or molecules in the gas they excite the species
In biology, a species is the basic unit of Taxonomy (biology), classification and a taxonomic rank of an organism, as well as a unit of biodiversity. A species is often defined as the largest group of organisms in which any two individuals of ...
to a higher energy level
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The ...
. In returning to their former energy levels these atoms or molecules release the energy that they had gained. That energy gets emitted as light. This light-producing process, called fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, ...
, causes a glow in the region behind the cathode.
Anode ray ion source
An anode ray ion source typically is an anode coated with thehalide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a f ...
salt of an alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of ...
or alkaline earth metal
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are ...
. Application of a sufficiently high electrical potential creates alkali or alkaline earth ions and their emission is most brightly visible at the anode.
See also
* Canal ray experimentsReferences
{{ReflistExternal links
Rays Of Positive Electricity by J.J. Thomson ''Proceedings of the Royal Society'', A 89, 1-20 (1913)
at The Cathode Ray Tube site German inventions Ionizing radiation Luminescence Mass spectrometry