Aminoacylase 2 on:
[Wikipedia]
[Google]
[Amazon]
Aspartoacylase is a hydrolytic enzyme (, also called ''aminoacylase II'', ''ASPA'' and other names) that in humans is encoded by the ''ASPA'' gene. ASPA

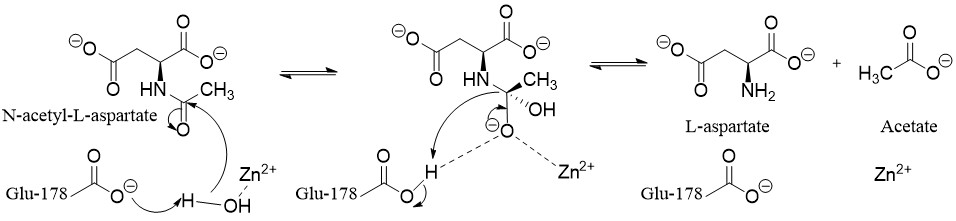

catalyzes
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
the deacylation of ''N''-acetyl-l-aspartate (''N-acetylaspartate)'' into aspartate
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the pro ...
and acetate
An acetate is a salt (chemistry), salt formed by the combination of acetic acid with a base (e.g. Alkali metal, alkaline, Alkaline earth metal, earthy, Transition metal, metallic, nonmetallic or radical Radical (chemistry), base). "Acetate" als ...
. It is a zinc-dependent hydrolase that promotes the deprotonation of water to use as a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
in a mechanism analogous to many other zinc-dependent hydrolases. It is most commonly found in the brain, where it controls the levels of ''N''-acetyl-l-aspartate. Mutations that result in loss of aspartoacylase activity are associated with Canavan disease
Canavan disease, or Canavan-Van Bogaert-Bertrand disease, is a rare and fatal autosomal recessive degenerative disease that causes progressive damage to nerve cells and loss of white matter in the brain. It is one of the most common degenerative ...
, a rare autosomal recessive
In genetics, dominance is the phenomenon of one variant (allele) of a gene on a chromosome masking or overriding the effect of a different variant of the same gene on the other copy of the chromosome. The first variant is termed dominant and t ...
neurodegenerative disease.
Structure
Aspartoacylase is adimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ( ...
of two identical monomers of 313 amino acids and uses a zinc cofactor in each. There are two distinct domains in each monomer: the N-terminal domain from residues 1-212 and the C-terminal domain from residues 213–313. The N-terminal domain of aspartoacylase is similar to that of zinc-dependent hydrolases such as carboxypeptidaseA. However, carboxypeptidases do not have something similar to the C-domain. In carboxypeptidase A, the active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) a ...
is accessible to large substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (locomotion), the surface over which an organism lo ...
s like the bulky C-terminal residue of polypeptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A p ...
s, whereas the C-domain sterically hinders access to the active site in aspartoacylase. Instead, the N-domain and C-domain of aspartoacylase form a deep narrow channel that leads to the active site.
The zinc cofactor is found at the active site and is held by Glu-24, His-21, and His 116. The substrate is held in place by Arg-63, Asn-70, Arg-71, Tyr-164, Arg-168, and Tyr-288. The zinc cofactor is used to lower the pKa
PKA may refer to:
* Professionally known as:
** Pen name
** Stage persona
* p''K''a, the symbol for the acid dissociation constant at logarithmic scale
* Protein kinase A, a class of cAMP-dependent enzymes
* Pi Kappa Alpha, the North-American so ...
of a ligated water molecule so that an attack on N-acetyl-L-aspartate may occur and to stabilize the resulting tetrahedral intermediate
A tetrahedral intermediate is a reaction intermediate in which the bond arrangement around an initially double-bonded carbon atom has been transformed from trigonal to tetrahedral. Tetrahedral intermediates result from nucleophilic addition to a c ...
along with Arg-63, and Glu-178.

Mechanism
There are two types of possible mechanisms for zinc-dependent hydrolases depending on what is thenucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
. The first uses deprotonated water and the second attacks with an aspartate
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the pro ...
or glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
first forming an anhydride. Aspartoacylase follows the deprotonated water mechanism. Zinc lowers the pKa of a ligated water molecule and the reaction proceeds via an attack on N-acetyl-l-aspartate when the water molecule is deprotonated by Glu-178. This leads to a tetrahedral intermediate
A tetrahedral intermediate is a reaction intermediate in which the bond arrangement around an initially double-bonded carbon atom has been transformed from trigonal to tetrahedral. Tetrahedral intermediates result from nucleophilic addition to a c ...
that is stabilized by the zinc, Arg-63, and Glu-178. Finally, the carbonyl is then reformed, the bond with nitrogen is broken, and the nitrogen is protonated by the proton taken by Glu-178 all in one concerted step.

Biological function
Aspartoacylase is used to metabolize ''N''-acetyl-L-aspartate by catalyzing its deacylation. Aspartoacylase prevents the buildup of N-acetyl-L-aspartate in the brain. It is believed that controlling N-acetyl-L-aspartate levels is essential for developing and maintaining white matter. It is not known why so much N-acetyl-L-aspartate is produced in the brain nor what its primary function is. However, one hypothesis is that it is potentially used as a chemical reservoir that can be tapped into for acetate foracetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for ...
synthesis or aspartate for glutamate synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
** Organic synthesis, the chemical synthesis of organ ...
. This way, N-acetyl-L-aspartate can be used to transport these precursor molecules and aspartoacylase is used to release them. For example, N-acetyl-L-aspartate produced in neurons can be transported into oligodendrocytes and the acetate released can be used for myelin synthesis. Another hypothesis is that N-acetyl-L-aspartate is essential osmolyte that acts as a molecular water pump that helps maintain a proper fluid balance in the brain.
Disease Relevance
Mutations that lead to loss of aspartoacylase activity have been identified as the cause ofCanavan disease
Canavan disease, or Canavan-Van Bogaert-Bertrand disease, is a rare and fatal autosomal recessive degenerative disease that causes progressive damage to nerve cells and loss of white matter in the brain. It is one of the most common degenerative ...
. Canavan disease is a rare autosomal recessive
In genetics, dominance is the phenomenon of one variant (allele) of a gene on a chromosome masking or overriding the effect of a different variant of the same gene on the other copy of the chromosome. The first variant is termed dominant and t ...
disorder that causes spongy degeneration of the white matter in the brain and severe psychomotor retardation, usually leading to death at a young age. The loss of aspartoacylase activity leads to the buildup of N-acetyl-L-aspartate in the brain and an increase in urine concentration by up to 60 times normal levels. Though the exact mechanism of how loss of aspartoacylase activity leads to Canavan disease is not fully understood, there are two primary competing explanations. The first is that it leads to defective myelin synthesis due to a deficiency of acetyl-CoA derived from the acetate product. Another explanation is that the elevated levels of N-acetyl-l-aspartate interfere with its normal brain osmoregulatory
Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration o ...
mechanism leading to osmotic disequilibrium.
There are over 70 reported mutations of this enzyme, but the most common ones are the amino acid substitutions E285A and A305E. E285A reduces activity of aspartoacylase down to as low as 0.3% of its normal function and is found in 98% of cases with Ashkenazi Jewish
Ashkenazi Jews ( ; he, יְהוּדֵי אַשְׁכְּנַז, translit=Yehudei Ashkenaz, ; yi, אַשכּנזישע ייִדן, Ashkenazishe Yidn), also known as Ashkenazic Jews or ''Ashkenazim'',, Ashkenazi Hebrew pronunciation: , singu ...
ancestry. The mutation A305E is found in about 40% of non-Jewish patients and reduces activity to about 10%. Of these two mutations, a crystal structure of the E285A mutant has been taken, showing that the loss of the hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ing from glutamate leads to a conformational change that distorts the active site and alters the substrate binding, leading to the much lower catalytic activity.
See also
*Aspartic acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the pro ...
*Neurodegeneration
A neurodegenerative disease is caused by the progressive loss of structure or function of neurons, in the process known as neurodegeneration. Such neuronal damage may ultimately involve cell death. Neurodegenerative diseases include amyotrophic ...
* Enzyme deficiency
Notes
References
External links
* * {{Enzymes EC 3.5.1