Amino Sugars on:
[Wikipedia]
[Google]
[Amazon]
In
 Azides give high
Azides give high 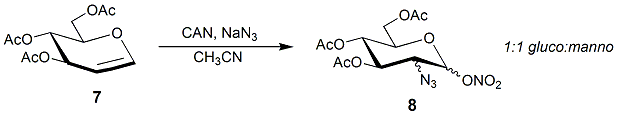 Glycals may also be converted into amino sugars by nitration followed by treatment with
Glycals may also be converted into amino sugars by nitration followed by treatment with  One-pot reactions have also been reported. For instance
One-pot reactions have also been reported. For instance
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
, an amino sugar (or more technically a 2-amino-2-deoxysugar) is a sugar molecule in which a hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydrox ...
group has been replaced with an amine group. More than 60 amino sugars are known, with one of the most abundant being ''N''-Acetyl--glucosamine, which is the main component of chitin
Chitin ( C8 H13 O5 N)n ( ) is a long-chain polymer of ''N''-acetylglucosamine, an amide derivative of glucose. Chitin is probably the second most abundant polysaccharide in nature (behind only cellulose); an estimated 1 billion tons of chit ...
.
Derivatives of amine containing sugars, such as ''N''-acetylglucosamine and sialic acid Sialic acids are a class of alpha-keto acid sugars with a nine-carbon backbone.
The term "sialic acid" (from the Greek for saliva, - ''síalon'') was first introduced by Swedish biochemist Gunnar Blix in 1952. The most common member of this ...
, whose nitrogens are part of more complex functional groups rather than formally being amines, are also considered amino sugars. Aminoglycosides are a class of antimicrobial compounds that inhibit bacterial protein synthesis. These compounds are conjugates of amino sugars and aminocyclitols.
Synthesis
From glycals
Glycal
Glycal is a name for cyclic enol ether derivatives of sugars having a double bond between carbon atoms 1 and 2 of the ring. The term “glycal” should not be used for an unsaturated sugar that has a double bond in any position other than betwee ...
s are cyclic enol ether
In organic chemistry an enol ether is an alkene with an alkoxy substituent. The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are vinyl ethers, with the formula ROCH=CH2. Important enol ethers includ ...
derivatives of monosaccharides
Monosaccharides (from Greek '' monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
They are usually colorless, water-sol ...
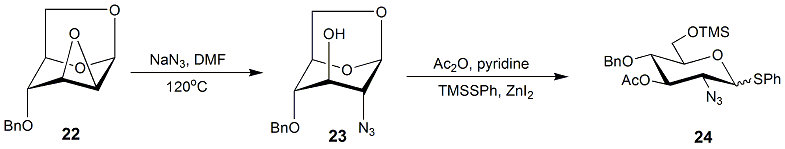
, having a double bond between carbon atoms 1 and 2 of the ring. ''N''-functionalized of glycals at the C2 position, combined with glycosidic bond formation at C1 is a common strategy for the synthesis of amino sugars. This can be achieved using azide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant ...
s with subsequent reduction yielding the amino sugar. One advantage of introducing azide moiety at C-2 lies in its non-participatory ability, which could serve as the basis of stereoselective synthesis of 1.2-cis-glycosidic linkage.
 Azides give high
Azides give high regioselectivity
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base ...
, however stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non-stereospecific creation of a new stereocenter or during a non-stereospecific transformation ...
both at C-1 and C-2 is generally poor. Usually anomeric
In carbohydrate chemistry, a pair of anomers () is a pair of near-identical stereoisomers that differ at only the anomeric carbon, the carbon that bears the aldehyde or ketone functional group in the sugar's open-chain form. However, in order for ...
mixtures will be obtained and the stereochemistry formed at C-2 is heavily dependent upon the starting substrates. For galactal, addition of azide to the double bond will preferentially occur from equatorial direction because of steric hindrance at the top face caused by axial group at C-4. For glucal, azide could attack from both axial and equatorial directions with almost similar probability, so its selectivity will decrease.
 Glycals may also be converted into amino sugars by nitration followed by treatment with
Glycals may also be converted into amino sugars by nitration followed by treatment with thiophenol
Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenol ...
(Michael addition) to furnish a thioglycoside donor. This is a versatile donor and can react with simple or carbohydrate alcohols to establish the glycosidic linkage, with reduction and ''N''-acetylation of nitro group will give the targeted product.
 One-pot reactions have also been reported. For instance
One-pot reactions have also been reported. For instance glycal
Glycal is a name for cyclic enol ether derivatives of sugars having a double bond between carbon atoms 1 and 2 of the ring. The term “glycal” should not be used for an unsaturated sugar that has a double bond in any position other than betwee ...
, activated by thianthrene-5-oxide and Tf2O is treated with an amide nucleophile and a glycosyl acceptor to produce various 1,2-trans C-2-amidoglycosides. Both the C-2 nitrogen introduction and the glycosidic bond formation precede stereoselectively. This methodology makes the introduction of both natural and non-natural amide functionalities at C-2 possible and more importantly with glycosidic bond formation at the same time in a one-pot procedure.
Via nucleophilic displacement
Nucleophilic displacement can be an effective strategy for the synthesis of amino sugars, however success strongly depends upon the nature of nucleophile, the type of leaving group and site of displacements on sugar rings. One aspect of this problem is that displacements at the C2 position tend to be slow as it is adjacent to theanomeric centre
In carbohydrate chemistry, a pair of anomers () is a pair of near-identical stereoisomers that differ at only the anomeric carbon, the carbon that bears the aldehyde or ketone functional group in the sugar's open-chain form. However, in order f ...
; this is particularly true for glycosides with axially-oriented aglycones.

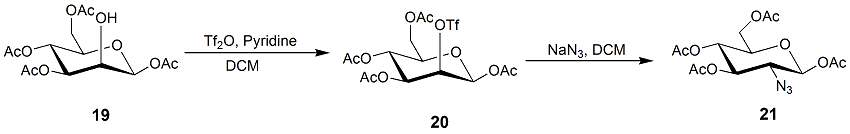
Epoxides
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale f ...
are suitable starting materials for realizing nucleophilic displacement reaction to introduce azide into C-2. Anhydrosugar 21 could be transformed into thioglycoside 22, which serves as a donor to react with alcohols to obtain 2-azide-2-deoxy-''O''-glycosides. The subsequent reduction and ''N''-acetylation will furnish the desired 2-''N''-acetamido-2-deoxyglycosides.

See also
*Iminosugar
An iminosugar, also known as an iminosaccharide, is any analog of a sugar where a nitrogen atom has replaced the oxygen atom in the ring of the structure.
Iminosugars are common components of plants and may be responsible for some of their medici ...
* ''N''-Acetylglucosamine
*Galactosamine
Galactosamine is a hexosamine derived from galactose with the molecular formula C6H13NO5. This amino sugar is a constituent of some glycoprotein hormones such as follicle-stimulating hormone (FSH) and luteinizing hormone (LH).
Precursors such a ...
*Glucosamine
Glucosamine (C6H13NO5) is an amino sugar and a prominent precursor in the biochemical synthesis of glycosylated proteins and lipids. Glucosamine is part of the structure of two polysaccharides, chitosan and chitin. Glucosamine is one of the mos ...
*Sialic acid Sialic acids are a class of alpha-keto acid sugars with a nine-carbon backbone.
The term "sialic acid" (from the Greek for saliva, - ''síalon'') was first introduced by Swedish biochemist Gunnar Blix in 1952. The most common member of this ...
*L- Daunosamine
* 1β-Methylseleno-N-acetyl-D-galactosamine
References
External links
* {{Authority control