Allylic Strain Acid Catalysis on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic
A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic

 In terms of
In terms of 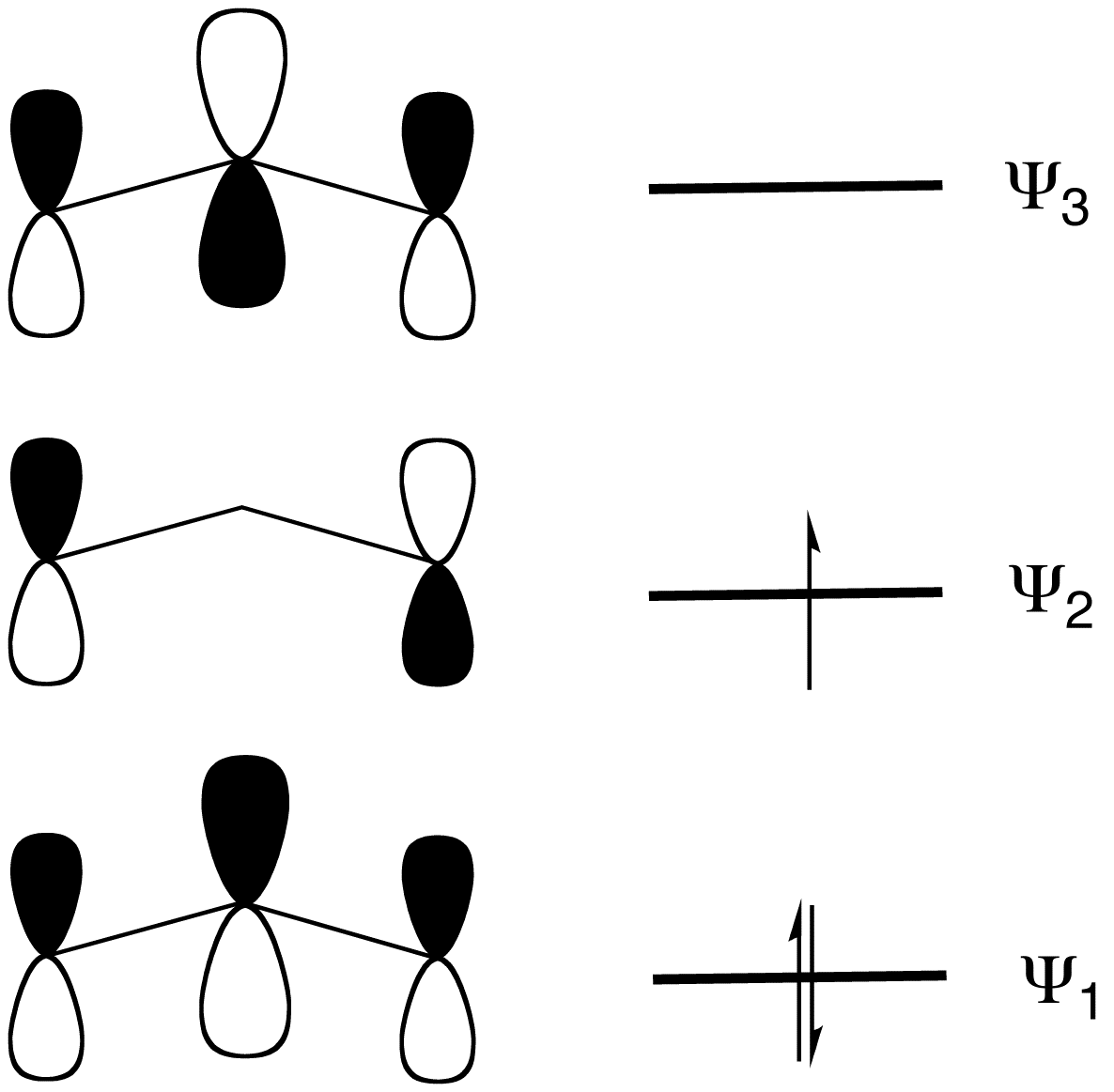
2CH3-CH=CH2 + 2 NH3 + 3 O2 -> 2CH2=CH-C#N + 6 H2O
An estimated 800,000 tonnes (1997) of CH3CH=CH2 + Cl2 -> ClCH2CH=CH2 + HCl
It is the precursor to
 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, an allyl group is a substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''side ...
with the structural formula
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are possibly arranged in the real three-dimensional space. The chemical bondi ...
, where R is the rest of the molecule. It consists of a methylene bridge
In organic chemistry, a methylene bridge, methylene spacer, or methanediyl group is any part of a molecule with formula ; namely, a carbon atom bound to two hydrogen atoms and connected by single bonds to two other distinct atoms in the rest of ...
() attached to a vinyl group
In organic chemistry, a vinyl group (abbr. Vi; IUPAC name: ethenyl group) is a functional group with the formula . It is the ethylene (IUPAC name: ethene) molecule () with one fewer hydrogen atom. The name is also used for any compound contain ...
(). The name is derived from the scientific name for garlic
Garlic (''Allium sativum'') is a species of bulbous flowering plant in the genus ''Allium''. Its close relatives include the onion, shallot, leek, chive, Allium fistulosum, Welsh onion and Allium chinense, Chinese onion. It is native to South A ...
, . In 1844, Theodor Wertheim
Theodor Wertheim (25 December 1820 – 6 July 1864) was an Austrian chemist born in Vienna. He was the father of gynecologist Ernst Wertheim (1864-1920).
He studied organic chemistry in Berlin as a pupil of Eilhard Mitscherlich, and in ...
isolated an allyl derivative from garlic oil
Garlic oil is the volatile oil derived from garlic. It is usually prepared using steam distillation and can also be produced via distillation using ether. It is used in cooking and as a seasoning, a nutritional supplement, and also as an insectic ...
and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride
Allyl chloride is the organic compound with the formula C H2=CHCH2 Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a ch ...
.
Allylation is any chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
that adds an allyl group to a substrate.
Nomenclature
hydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy g ...
". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive.
Benzylic
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group.
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a substi ...
and allylic are related in terms of structure, bond strength, and reactivity. Other reactions that tend to occur with allylic compounds are allylic oxidation
Organoselenium compounds (or seleno-organic) are chemical compounds containing carbon-to-selenium chemical bonds. Organoselenium chemistry is the corresponding science exploring their properties and reactivity. Selenium belongs with oxygen and sulf ...
s, ene reaction
In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile ...
s, and the Tsuji–Trost reaction
The Tsuji–Trost reaction (also called the Trost allylic alkylation or allylic alkylation) is a palladium-catalysed substitution reaction involving a substrate that contains a leaving group in an allylic position. The palladium catalyst first coo ...
. Benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group.
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a substi ...
ic groups are related to allyl groups; both show enhanced reactivity.
Pentadienyl group
A group connected to two vinyl groups is said to be doubly allylic. Thebond dissociation energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical s ...
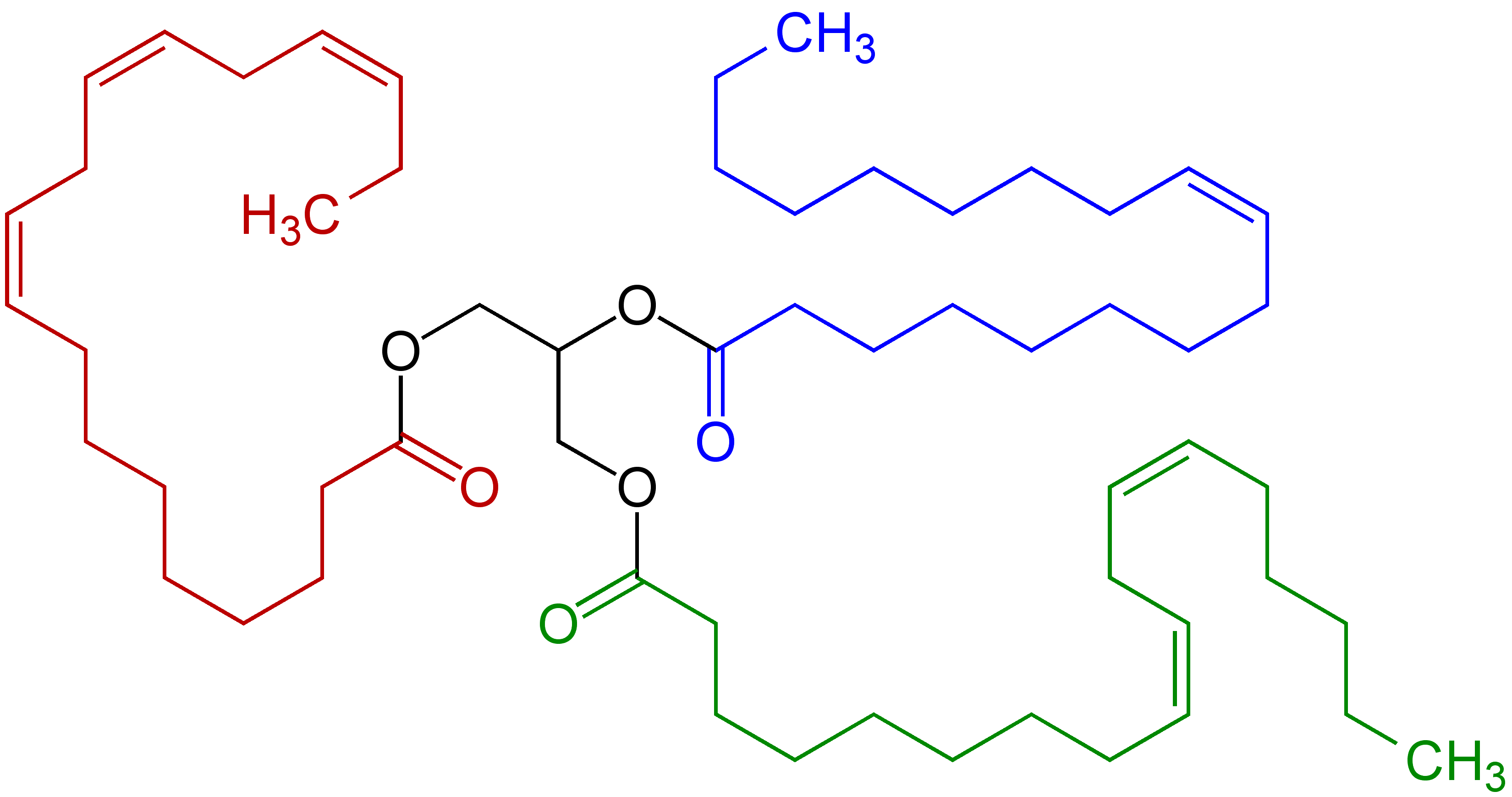
of C−H bonds on a doubly allylic centre is about 10% less than the bond dissociation energy of a C−H bond that is allylic. The weakened C−H bonds reflect the high stability of the resulting pentadienyl
In organic chemistry, pentadienyl refers to the organic radical, anion, or cation with the formula , where ''z'' = 0, −1, +1, respectively.
Organometallic chemistry
In organometallic chemistry, the pentadienyl anion is a ligand, the acyclic ana ...
radicals. Compounds containing the linkages, e.g. linoleic acid
Linoleic acid (LA) is an organic compound with the formula COOH(CH2)7CH=CHCH2CH=CH(CH2)4CH3. Both alkene groups are cis-trans isomerism, ''cis''. It is a fatty acid sometimes denoted 18:2 (n-6) or 18:2 ''cis''-9,12. A linoleate is a salt (chem ...
derivatives, are prone to autoxidation, which can lead to polymerization or form semisolids. This reactivity pattern is fundamental to the film-forming behavior of the "drying oil
A drying oil is an oil that hardens to a tough, solid film after a period of exposure to air, at room temperature. The oil hardens through a chemical reaction in which the components crosslink (and hence, polymerize) by the action of oxygen (not ...
s", which are components of oil paint
Oil paint is a type of slow-drying paint that consists of particles of pigment suspended in a drying oil, commonly linseed oil. The viscosity of the paint may be modified by the addition of a solvent such as turpentine or white spirit, and varn ...
s and varnish
Varnish is a clear transparent hard protective coating or film. It is not a stain. It usually has a yellowish shade from the manufacturing process and materials used, but it may also be pigmented as desired, and is sold commercially in various ...
es.

Homoallylic
The term homoallylic refers to the position on a carbon skeleton next to an allylic position. In but-3-enyl chloride , the chloride is homoallylic because it is bonded to the homoallylic site.
Bonding
The allyl group is widely encountered in organic chemistry.Jerry March, "Advanced Organic Chemistry" 4th Ed. J. Wiley and Sons, 1992: New York. . Allylic radicals,anions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
, and cations
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
are often discussed as intermediates in reactions. All feature three contiguous sp²-hybridized carbon centers and all derive stability from resonance.Organic Chemistry John McMurry 2nd ed. 1988 Each species can be presented by two resonance structures
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
with the charge or unpaired electron distributed at both 1,3 positions.
:MO theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century.
In molecular orbital theory, electrons in a molecul ...
, the MO diagram
Mo or MO may refer to:
Arts and entertainment Fictional characters
* Mo, a girl in the ''Horrible Histories'' TV series
* Mo, also known as Mortimer, in the novel ''Inkheart'' by Cornelia Funke
* Mo, in the webcomic '' Jesus and Mo''
* Mo, the ...
has three molecular orbitals: the first one bonding, the second one non-bonding, and the higher energy orbital is antibonding.Organic Chemistry 4th Ed. Morisson & Boyd 1988.
:
Reactions and applications
This heightened reactivity of allylic groups has many practical consequences. Thesulfur vulcanization
Sulfur vulcanization is a chemical process for converting natural rubber or related polymers into materials of varying hardness, elasticity, and mechanical durability by heating them with sulfur or sulfur-containing compounds. Sulfur forms cros ...
or various rubbers exploits the conversion of allylic groups into crosslinks. Similarly drying oil
A drying oil is an oil that hardens to a tough, solid film after a period of exposure to air, at room temperature. The oil hardens through a chemical reaction in which the components crosslink (and hence, polymerize) by the action of oxygen (not ...
s such as linseed oil
Linseed oil, also known as flaxseed oil or flax oil (in its edible form), is a colourless to yellowish oil obtained from the dried, ripened seeds of the flax plant (''Linum usitatissimum''). The oil is obtained by pressing, sometimes followed by ...
crosslink via oxygenation of allylic (or doubly allylic) sites. This crosslinking underpins the properties of paints and the spoilage of foods by rancidification
Rancidification is the process of complete or incomplete autoxidation or hydrolysis of fats and oils when exposed to air, light, moisture, or bacterial action, producing short-chain aldehydes, ketones and free fatty acids.
When these processes oc ...
.
The industrial production of acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecular ...
by ammoxidation
In organic chemistry, ammoxidation is a process for the production of nitriles () using ammonia () and oxygen (). It is sometimes called the SOHIO process, acknowledging that ammoxidation was developed at Standard Oil of Ohio. The usual substrate ...
of propene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula CH3CH=CH2. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petrole ...
exploits the easy oxidation of the allylic C−H centers:
:allyl chloride
Allyl chloride is the organic compound with the formula C H2=CHCH2 Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a ch ...
is produced by the chlorination Chlorination may refer to:
* Chlorination reaction
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transform ...
of propylene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula CH3CH=CH2. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petrole ...
:
:allyl alcohol
Allyl alcohol (IUPAC name: prop-2-en-1-ol) is an organic compound with the structural formula . Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a raw material ...
and epichlorohydrin
Epichlorohydrin (abbreviated ECH) is an organochlorine compound and an epoxide. Despite its name, it is not a halohydrin. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar organi ...
.
Allylation
Allylation is the attachment of an allyl group to a substrate, usually another organic compound. Classically, allylation involves the reaction of acarbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3C ...
with allyl chloride. Another well-developed process involves addition of allyltrimethylsilane
Allyltrimethylsilane is the organosilicon compound
Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organo ...
to carbonyls, i.e. carbonyl allylation In organic chemistry, carbonyl allylation describes methods for adding an allyl anion to an aldehyde or ketone to produce a homoallylic alcohol. The carbonyl allylation was first reported in 1876 by Alexander Zaitsev and employed an allylzinc reage ...
.
Allylation can be effected also by conjugate addition
Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarit ...
: the addition of an allyl group to the beta-position of an enone. The Hosomi-Sakurai reaction is a common method for conjugate allylation.

Allyl compounds
Many substituents can be attached to the allyl group to give stable compounds. Commercially important allyl compounds include: *Crotyl alcohol
Crotyl alcohol, or crotonyl alcohol, is an unsaturated alcohol. It is a colourless liquid that is moderately soluble in water and miscible with most organic solvents. Two isomers of this alcohol exist, cis and trans.
It can be synthesized by th ...
(CH3CH=CH−CH2OH)
*Dimethylallyl pyrophosphate
Dimethylallyl pyrophosphate (DMAPP; or alternatively, dimethylallyl diphosphate (DMADP); also isoprenyl pyrophosphate) is an isoprenoid precursor. It is a product of both the mevalonate pathway and the MEP pathway of isoprenoid precursor biosynt ...
, central in the biosynthesis of terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes ar ...
s, a precursor to many natural products, including natural rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, and ...
.
*Transition-metal allyl complex
Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usual ...
es, such as allylpalladium chloride dimer
Allylpalladium(II) chloride dimer (APC) is a chemical compound with the chemical formula, formula hapticity, η3-C3H5)PdCl. This yellow air-stable compound is an important catalyst used in organic synthesis.Tatsuno, Y.; Yoshida, T.; Otsuka, S. ...
See also
*Allylic strain
250 px , right , Allylic strain in an olefin.
Allylic strain (also known as A1,3 strain, 1,3-allylic strain, or A-strain) in organic chemistry is a type of strain energy resulting from the interaction between a substituent on one end of an olef ...
* Carroll rearrangement
* Allylic palladium complex
* Tsuji–Trost reaction
The Tsuji–Trost reaction (also called the Trost allylic alkylation or allylic alkylation) is a palladium-catalysed substitution reaction involving a substrate that contains a leaving group in an allylic position. The palladium catalyst first coo ...
* Propargylic/Homopropargylic
* Benzylic
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group.
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a substi ...
* Vinylic
In organic chemistry, a vinyl group (abbr. Vi; IUPAC name: ethenyl group) is a functional group with the formula . It is the ethylene (IUPAC name: ethene) molecule () with one fewer hydrogen atom. The name is also used for any compound contai ...
* Acetylenic In organic chemistry, the term acetylenic designates
*A doubly unsaturated position (''sp''-hybridized) on a molecular framework, for instance in an alkyne such as acetylene;
*An ethynyl fragment, HC\equivC–, or substituted homologue.
See also ...
* Naloxone
Naloxone, sold under the brand names Narcan (4 mg) and Kloxxado (8 mg) among others, is a medication used to reverse or reduce the effects of opioids. It is commonly used to counter decreased breathing in opioid overdose. Effects begin within ...
* Allylic rearrangement An allylic rearrangement or allylic shift is an organic reaction in which the double bond in an allyl chemical compound shifts to the next carbon atom. It is encountered in nucleophilic substitution.
In reaction conditions that favor a SN1 react ...
References
{{Authority control Alkenyl groups Allyl compounds