Adatom on:
[Wikipedia]
[Google]
[Amazon]
 An adatom is an
An adatom is an
atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
that lies on a crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
surface, and can be thought of as the opposite of a surface vacancy. This term is used in surface chemistry
Surface science is the study of physics, physical and chemistry, chemical phenomena that occur at the interface (chemistry), interface of two phase (matter), phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum int ...
and epitaxy
Epitaxy (prefix ''epi-'' means "on top of”) is a type of crystal growth or material deposition in which new crystalline layers are formed with one or more well-defined orientations with respect to the crystalline seed layer. The deposited cry ...
, when describing single atoms lying on surfaces and surface roughness
Surface roughness or simply roughness is the quality of a surface of not being smooth and it is hence linked to human ( haptic) perception of the surface texture. From a mathematical perspective it is related to the spatial variability structure ...
. The word is a portmanteau
In linguistics, a blend—also known as a blend word, lexical blend, or portmanteau—is a word formed by combining the meanings, and parts of the sounds, of two or more words together.
of " adsorbed atom". A single atom, a cluster of atoms, or a molecule or cluster of molecules may all be referred to by the general term " adparticle". This is often a thermodynamically unfavorable state. However, cases such as graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
may provide counter-examples.
Growth
″Adatom″ is aportmanteau word
In linguistics, a blend—also known as a blend word, lexical blend, or portmanteau—is a word formed by combining the meanings, and parts of the sounds, of two or more words together.) Israeli שלט ''shalát'' 'remote control', an ellipsis ...
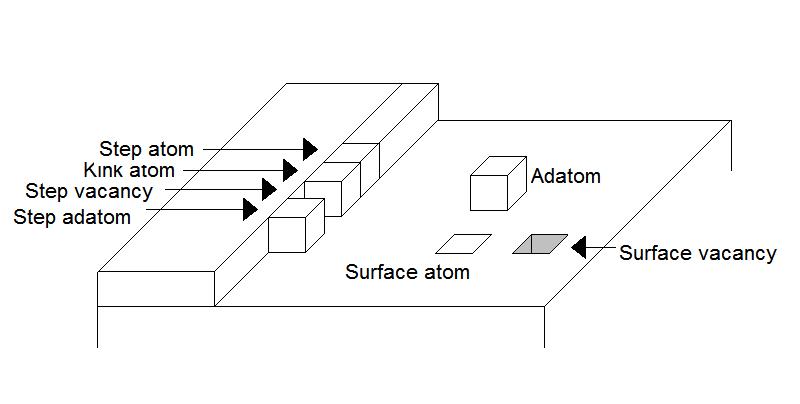
, short for adsorbed atom. When the atom arrives at a crystal surface, it is adsorbed by the periodic potential of the crystal, thus becoming an adatom. The minima of this potential form a network of adsorption sites on the surface. There are different types of adsorption sites. Each of these sites corresponds to a different structure of the surface. There are five different types of adsorption sites, which are: on a terrace, where the adsorption site is on top of the surface layer that is growing; at the step edge, which is next to the growing layer; in the kink of a growing layer; in the step edge of a growing layer, and in the surface layer, where the adsorption site is inside the lower layer.
Out of these adsorption site types, kink sites play the most important role in crystal growth
Crystal growth is a major stage of a crystallization, crystallization process, and consists of the addition of new atoms, ions, or polymer strings into the characteristic arrangement of the crystalline lattice. The growth typically follows an ini ...
. Kink density is a major factor of growth kinetics. Attachment of an atom to the kink site, or removal of the atom from the kink, does not change the free surface energy
In surface science, surface energy (also interfacial free energy or surface free energy) quantifies the disruption of intermolecular bonds that occurs when a surface is created. In solid-state physics, surfaces must be intrinsically less energe ...
of the crystal, since the number of broken bonds does not change. This gives that the chemical potential
In thermodynamics, the chemical potential of a Chemical specie, species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potent ...
of an atom in the kink site is equal to that of the crystal, which means that the kink site is the one adsorption site type where an adatom becomes a part of the crystal.
If crystallography
Crystallography is the branch of science devoted to the study of molecular and crystalline structure and properties. The word ''crystallography'' is derived from the Ancient Greek word (; "clear ice, rock-crystal"), and (; "to write"). In J ...
is used, or if the growth temperatures are higher, which would give an entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the micros ...
effect, the crystal surface becomes rough, causing greater number of kinks. This means that adatoms have a greater chance of arriving at a kink site, to become part of the crystal. This is the normal mechanism of growth.
The opposite, so with a lower growth temperature, would give a smooth surface, which means that there is a higher number of terrace adsorption sites. There are still kink sites, but these are only found at the edges of steps. The crystal only grows through "lateral motion of the steps". This type of growth is called the layer mechanism of growth. How the adatoms grow on the surface depends on what interaction is the strongest or what the surface looks like. If the adatom-adatom interaction is the strongest, adatoms are more likely to create pyramids of adatoms on the surface. If the adatom-surface interaction is the strongest, the adatoms are more likely to arrange themselves in such a way as to create layers on the surface. But it also depends on the origins of the steps on the surface. In total there are five different types of layer growth: normal growth, step-flow growth, layer-by-layer growth, multilayer (or three-dimensional island) growth, and spiral growth.
Step-flow growth is observed on stair-like surfaces. These surfaces have a geometry with vicinal steps separated by "atomically flat low-index terraces". When adatoms attach to the edges of the steps, they move along the surface, until they find a kink site to attach to become part of the crystal. However, if the kink density is not high enough, and thus not all adatoms arrive at one of the kinks, additional steps, as if there is a flat surface with small two-dimensional islands on it, are created on the terraces, leading to a mixed growth mode, which leads to a change in layer growth type, from step-flow to layer-by-layer growth.
In layer-by-layer growth, the adatom-surface interaction is the strongest. A new layer is created through 2D islands, which is created on the surface. The islands grow until they spread out over the entire surface, and the next layer will start to grow. This growth is named Frank–Van der Merwe growth
Frank–Van der Merwe growth (FM growth) is one of the three primary modes by which thin films grow epitaxially at a crystal surface or interface. It is also known as 'layer-by-layer growth'. It is considered an ideal growth model, requiring perf ...
.
In some cases the cycle of making new layers in layer-by-layer growth is broken by kinetic constraints. In these cases, growth in higher layers starts before lower layers are finished, which means three-dimensional islands are created. A new type of growth, called multilayer growth, is started, instead of the layer-by-layer growth. Multilayer growth can be divided into Volmer-Weber growth and Stranski-Krastanov growth.
If the crystal surface contains a screw dislocation
In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to sli ...
, a different type of growth, called spiral growth might take place. Around the screw dislocation, a spiral shape is seen during growth. As the screw dislocation causes a growth spiral that does not disappear, islands might not be needed to cause crystal growth.
The adatoms are bound to the surface through epitaxy. In this process, new layers of a crystal are created through the attachment of new atoms. This can be through a chemical reaction, or through heating a new film or centrifuging it. Generally, what happens is that the particles that are used to form a new layer, will not always be adsorbed. To create bonds with the surface, energy is needed and not every particle has the needed amount of energy to attach at that part of the surface (for different parts, different energies are needed). If one has a flux ''F'' of particles incoming, part of it will be adsorbed, given by the adsorption flux
:
where ''s'' here is the sticking coefficient. Not only does this variable depend on the surface and on the energy of the incoming atom, but also on the chemical nature of both the particle and the surface. If both the particle and surface are made of a substance that easily reacts with other particles, it is easier for the atoms to stick to the surface.
Surface thermodynamics
Taking a look at the thermodynamics at the surface of the film, it is seen that bonds are broken, releasing energy, and bonds are formed, confining energy. The thermodynamics involved were modeled by Walther Kossel and Ivan Stranski in 1920. This model is calledterrace ledge kink model In chemistry, the terrace ledge kink (TLK) model, which is also referred to as the terrace step kink (TSK) model, describes the thermodynamics of crystal surface formation and transformation, as well as the energetics of surface defect formation. It ...
(TLK).
The adatom can create more than one bond with the crystal, depending on the structure of the crystal. If it is a simple cubic lattice, the adatom can have up to 6 bonds, whereas in a face-centered cubic lattice, it can have up to 12 nearest neighbors. The more bonds created, the more energy is confined, making it harder to desorb the adatom.
A special site for an adatom is a kink, where exactly half of the bonds with the surface can be created, also called the "half-crystal position".
Magnetic adatoms
Adatoms, due to having fewer bonds than the other atoms in the crystal, have unboundelectron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s. These electrons have spin and therefore a magnetic moment
In electromagnetism, the magnetic moment or magnetic dipole moment is the combination of strength and orientation of a magnet or other object or system that exerts a magnetic field. The magnetic dipole moment of an object determines the magnitude ...
. This magnetic moment has no preference for orientation until an external influence, like a magnetic field
A magnetic field (sometimes called B-field) is a physical field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular ...
, is present. The structure of the adatoms on a surface can be adjusted by changing the external magnetic field. Through this method theoretical situations, such as the atomic chain, can be simulated. Quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
needs to be taken into account when using adatoms due to the small scale.
The magnetic field created by an atom is caused mostly by the orbit and spin of the electrons. The proton's and neutron's magnetic moment are negligible when compared to that of the electron due to their larger masses. When an atom with free electrons is inside an external magnetic field, its magnetic moment aligns with the external field because this lowers its energy. This is why bound electrons do not display this magnetic moment, they already have a favorable energy state and it is unfavorable to change. The magnetization
In classical electromagnetism, magnetization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material. Accordingly, physicists and engineers usually define magnetization as the quanti ...
of an (magnetically aligned) atom is given by:
:
Where ''N'' is the number of electrons, ''g''j is the g-factor, ''μ''B is the Bohr magneton
In atomic physics, the Bohr magneton (symbol ) is a physical constant and the natural unit for expressing the magnetic moment of an electron caused by its orbital or spin angular momentum.
In SI units, the Bohr magneton is defined as
\mu_\mat ...
, ''k''B is the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the ...
, ''T'' is the temperature and ''j'' is the total angular momentum quantum number. This formula holds under the assumption that the magnetic energy of an electron is given by and there is no exchange interaction
In chemistry and physics, the exchange interaction is a quantum mechanical constraint on the states of indistinguishable particles. While sometimes called an exchange force, or, in the case of fermions, Pauli repulsion, its consequences cannot alw ...
.
Movement across a surface
The movement of adatoms across a surface can be described by the Burton–Cabrera–Frank (CBF) model by Keith Burton, Nicolás Cabrera and Charles Frank. The model treats adatoms as a 2D gas on top of the surface. The adatoms diffuse with a diffusion constant ''D''; they are desorbed back to the medium above with a rate of per atom and adsorbed with flux ''F''. The diffusion constant can be, when the concentration of particles is small, expressed as: : Where ''a'' is the hopping distance for the atom. ''E''D is the energy needed to pass the diffusion barrier. ''ν''0 is the attempt frequency. The CBF model obeys the followingcontinuity equation
A continuity equation or transport equation is an equation that describes the transport of some quantity. It is particularly simple and powerful when applied to a conserved quantity, but it can be generalized to apply to any extensive quantity ...
:
:
Combining the steady states () with the following boundary conditions can lead to an expression for the velocity of the adatoms at each adsorption site.
: