ATP6V1G2 on:
[Wikipedia]
[Google]
[Amazon]
V-type 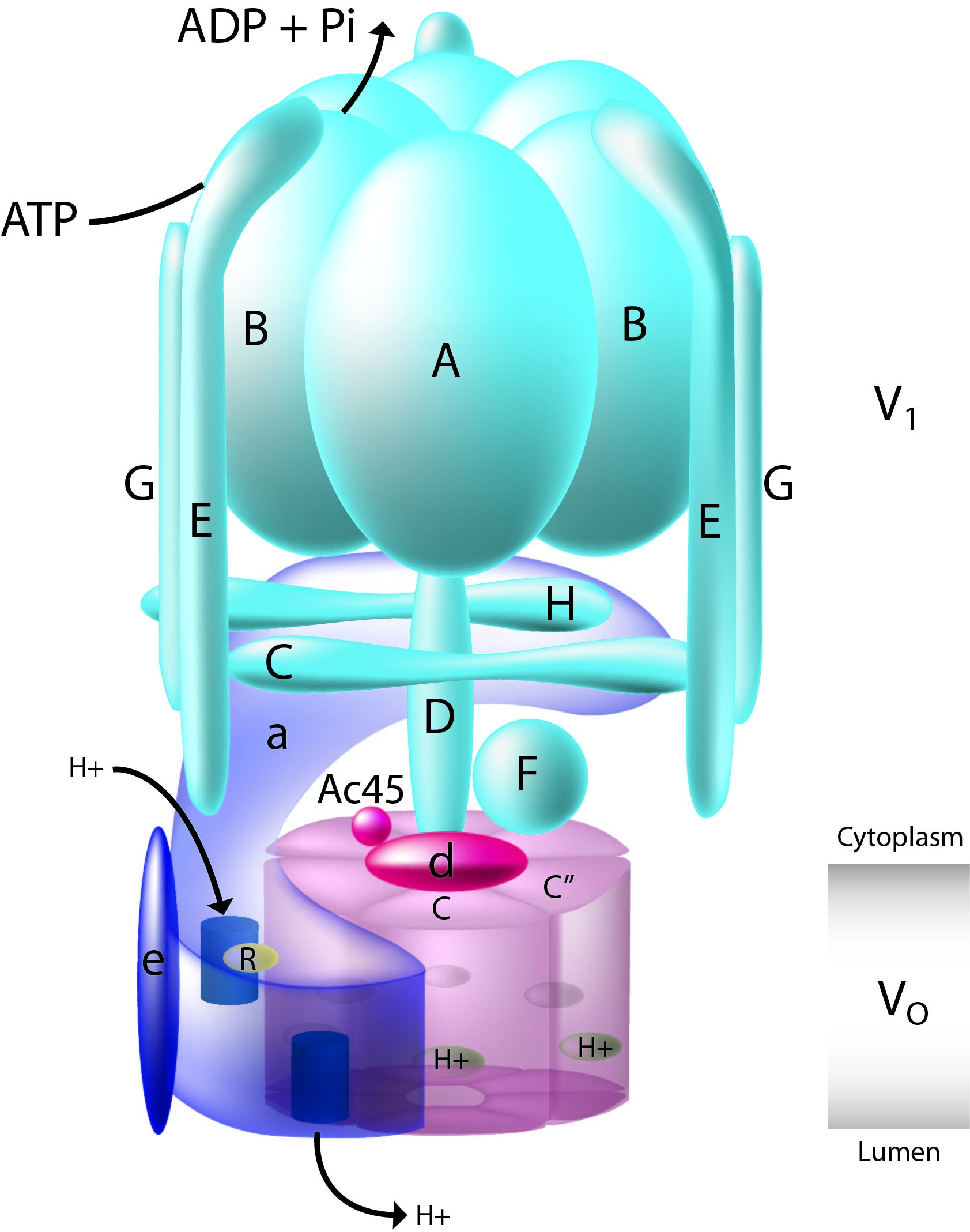 This gene encodes a component of
This gene encodes a component of
proton ATPase
In the field of enzymology, a proton ATPase is an enzyme that catalyzes the following chemical reaction:
:ATP + + in \rightleftharpoons ADP + phosphate + out
The 3 substrates of this enzyme are ATP, , and , whereas its 3 products are ADP, ph ...
subunit G 2 is an enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
that in human
Humans (''Homo sapiens'') are the most abundant and widespread species of primate, characterized by bipedalism and exceptional cognitive skills due to a large and complex brain. This has enabled the development of advanced tools, culture, ...
s is encoded by the ''ATP6V1G2'' gene
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a ba ...
.
 This gene encodes a component of
This gene encodes a component of vacuolar ATPase
Vacuolar-type ATPase (V-ATPase) is a highly conserved evolutionarily ancient enzyme with remarkably diverse functions in eukaryotic organisms. V-ATPases acidify a wide array of intracellular organelles and pumps protons across the plasma ...
(V-ATPase), a multisubunit enzyme that mediates acidification of intracellular compartments of eukaryotic
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
cells. V-ATPase dependent acidification is necessary for such intracellular processes as protein sorting
:''This article deals with protein targeting in eukaryotes unless specified otherwise.''
Protein targeting or protein sorting is the biological mechanism by which proteins are transported to their appropriate destinations within or outside the ce ...
, zymogen
In biochemistry, a zymogen (), also called a proenzyme (), is an inactive precursor of an enzyme. A zymogen requires a biochemical change (such as a hydrolysis reaction revealing the active site, or changing the configuration to reveal the active ...
activation, receptor-mediated endocytosis
Endocytosis is a cellular process in which substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a vesicle containing the ingested material. E ...
, and synaptic vesicle
In a neuron, synaptic vesicles (or neurotransmitter vesicles) store various neurotransmitters that are released at the synapse. The release is regulated by a voltage-dependent calcium channel. Vesicles are essential for propagating nerve impulse ...
proton gradient
An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consists of two parts, the chemical gradient, or difference in solute concentration across a membrane, and th ...
generation. V-ATPase is composed of a cytosolic
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells (intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrio ...
V1 domain and a transmembrane V0 domain. The V1 domain consists of three A and three B subunits, two G subunits plus the C, D, E, F, and H subunits. The V1 domain contains the ATP catalytic
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
site. The V0 domain consists of five different subunits: a, c, c', c double prime, and d.
Additional isoform
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene or gene family and are the result of genetic differences. While many perform the same or similar biological roles, some isof ...
s of many of the V1 and V0 subunit proteins are encoded by multiple genes, or alternatively spliced transcript variants. This encoded protein is one of three V1 domain G subunit proteins. This gene had previous gene symbol
Gene nomenclature is the scientific naming of genes, the units of heredity in living organisms. It is also closely associated with protein nomenclature, as genes and the proteins they code for usually have similar nomenclature. An international co ...
s of ATP6G and ATP6G2. Alternatively spliced transcript variants encoding different isoforms have been described.
Subcellular and tissue distribution
ATP6V1G2 is a subunit of a protein that has been identified in cell membranes, including intracellular membranes. These include lysosome, vacuole, and vesicle membranes within the cell. ATP6V1G2 is mostly found in the brain, and a smaller amount is found in the adrenals. The enzyme is coded for by 4 exons. The enzyme serves three main functions within the cell. First, ATP is hydrolyzed by this enzyme. This means that ATP is broken down with water into ADP and a Hydrogen ion. This Hydrogen ion serves as energy to drive other processes. ATP6V1G2 also allows other proteins to bind within the cell. ATP6V1G2 also allows ATPase to work by bringing Hydrogen ions into the cell.Structure
ATP6V1G2 is made of 118 amino acids. ATP6V1G2 can make the pH of certain areas lower.Function
The biochemical abilities of ATP6V1G2 are involved in two other processes. The first one includes managing autophagy within the cell. The second is the decrease in pH of vesicles in the synapse. ATP6V1G2 is the specific part of the enzyme that hydrolyses ATP as a peripheral protein. There are approximately 45,000 ATP6V1G2 proteins in the membrane, with "150 proton pumps per" micrometers squared. ATP6V1G2 is a subunit of a protein. It is in eukaryotic cells. It pumps protons. It is usually in internal plasma membranes. The V1 portion of the protein "is an ATPase" and is outside of the plasma membrane. ATP is required for hydrogens to enter the vesicle. The V0 part of the protein is in the plasma membrane and the "a subunit" is used to determine the isoform of the protein. Different isoforms are found in different tissues in mammals. The release of V1 from V0 after protons have entered the vesicle and neurotransmitters, allows the V0 domain to travel with the vesicle to bind to another V0 domain and transfer the neurotransmitters. There is a set amount of G2 and G1 subunits of the protein. ATP6V1G2 has significant functions regarding the function of nerve signals. The acidification of the interior of vesicles by ATP6V1G2 creates a difference in pH that is required for the neurotransmitters to enter the vesicle. In this way, ATP6V1G2 allows for the preparation of neurotransmitters in the nerve vesicle. The decrease in pH by ATP6V1G2 in the vesicle is important in the function of vesicle binding to the SNARE protein and endocytosis. Activation of a nerve causes lower pH in the vesicles, and a larger pH in the cell. Calcium and hydrogen antiporters are required for ATP6V1G2 to acidify the inside of the vesicle. The calcium is required to exit the cell in order to increase the number of hydrogens. ATP6V1G2 lowers the proton concentration in the cell by the vesicle binding to the plasma membrane and lowering the concentration of the protons. The ATP6V1G2 functions to prepare the vesicle for neurotransmitters to enter the vesicle, the binding of the vesicle to the plasma membrane, and the endocytosis of the vesicle. vATPase helps in the process of neurotransmitters being brought into the vesicle and in the binding of the vesicle to the synapse. The ATP6V1G2 part of the vATPase offers catalytic ability. ATP6V1G2 is needed for function, however, no abnormalities were seen in its absence in the experiment of turning off the gene in mice. ATP6V1G1 was increased when ATP6V1G2 was not present. There was not more mRNA with the absence of ATP6V1G2. More ATP6V1G1 was made without increased transcription. ATP6V1G2 completes processes involved with moving substances within the cell, as well as membranes, and the digestion of food. The functioning of vATPase is required for life. The processes of vATPase allows for the immune cells to remove microorganisms, by the macrophage. The vATPase also allows for T-cells and antigens to function. The vATPase is also involved in acidifying the extracellular area of "bone resorbing osteoclasts," and "epithelial cells in the kidney." The ATP6V1G2 is the part of the protein that connects the V1 and V0 components of the protein together. The ATP6V1G2 is required for the "energy coupling" between V1 and V0, allowing V1 and V0 to attach or unattach. The G component of vATPase weighs 13 kDa. ATP6V1G2 is found in the brain. The experiment of creating a nonfunctional ATP6V1G2 gene in mice created no effects for offspring. ATP6V1G2 is involved in ATP hydrolysis. ATP6V1G2 was not able to substitute for Vma10 decrease, while ATP6V1G1 was.Clinical significance
Dysfunction of ATP6V1G2 leads to various disorders. These can include "Noonan Syndrome 9," "Distal Renal Tubular Acidosis," and "Noonan Syndrome 3.". ATP6V1G2 may be involved in autoimmune diseases. Activated macrophages created more ATP6V1G2. The ATP6V1G2 gene is found in a 122kb group in the "TNF locus." The increased ATP6V1G2 was independent of TNF. The mRNA determines how much ATP6V1G2 is made because of an activated macrophage. ATP6V1G2 may be a result of the inflammatory response. ATP6V1G2 is involved with "protein sorting and degradation," "generation of secretory granules and endocytosis, and is known to be important for inflammatory and immune cell differentiation and function." Factors like increased salt can increase vATPase. Neutrophils, "degranulation," and "phagocytosis" from protein kinase C increases vATPase. vATPase activity creates immune response against pathogens by macrophage activation. Dendritic cells require vATPase to mature.References
Further reading
* * * * * * * * * * *External links
* {{UCSC gene info, ATP6V1G2