4-hydroxybenzoate 3-monooxygenase on:
[Wikipedia]
[Google]
[Amazon]
The 
 The hydroxylase, 4-hydroxybenzoate 3-monooxygenase, proceeds through a catalytic process that begins with the entrance of
The hydroxylase, 4-hydroxybenzoate 3-monooxygenase, proceeds through a catalytic process that begins with the entrance of
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
4-hydroxybenzoate 3-monooxygenase, also commonly referred to as para-hydroxybenzoate hydroxylase (PHBH), is a flavoprotein belonging to the family of oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually ut ...
s. Specifically, it is a hydroxylase
In chemistry, hydroxylation can refer to:
*(i) most commonly, hydroxylation describes a chemical process that introduces a hydroxyl group () into an organic compound.
*(ii) the ''degree of hydroxylation'' refers to the number of OH groups in a m ...
, and is one of the most studied enzymes and catalyzes
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
reactions involved in soil detoxification, metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cell ...
, and other biosynthetic processes.
4-hydroxybenzoate 3-monooygenase catalyzes the regioselective hydroxylation of 4-hydroxybenzoate
4-Hydroxybenzoic acid, also known as ''p''-hydroxybenzoic acid (PHBA), is a monohydroxybenzoic acid, a phenolic derivative of benzoic acid. It is a white crystalline solid that is slightly soluble in water and chloroform but more soluble in polar ...
, giving 3,4-dihydroxybenzoate
Protocatechuic acid (PCA) is a dihydroxybenzoic acid, a type of phenolic acid. It is a major metabolite of antioxidant polyphenols found in green tea. It has mixed effects on normal and cancer cells in ''in vitro'' and ''in vivo'' studies.
Biolo ...
as the product. The mechanism
Mechanism may refer to:
*Mechanism (engineering), rigid bodies connected by joints in order to accomplish a desired force and/or motion transmission
*Mechanism (biology), explaining how a feature is created
*Mechanism (philosophy), a theory that a ...
consists of the following general steps: (1) reduction of the flavin, (2) reaction of the flavin with O2, producing C4a-hydroperoxyflavin, and (3) binding and activation of the substrate, leading to product formation and release. Throughout the mechanism, the flavin changes between “open” and “closed” conformations, thus altering the substrate reaction environment. The open conformation allows solvent access to the active site; the enzyme adopts this conformation for substrate binding and product release. A closed conformation isolates the reaction from solvent, which helps to stabilize the reaction intermediates.

Structure
4-hydroxybenzoate 3-monooxygenase is a homodimer with a flavin bound to eachmonomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
Mo ...
. The active site is composed of the flavin and amino acids on the monomer. The structure of this enzyme often serves as a model for structure-reactivity interdependence of other flavin-dependent hydroxylases. The active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) a ...
limits potential substrates to substituted benzenes, namely 4-hydroxybenzoate (the native substrate), 2,4-dihydroxybenzoate, 4-mercaptobenzoate, and several halogenated aromatic compounds.
Mechanism
 The hydroxylase, 4-hydroxybenzoate 3-monooxygenase, proceeds through a catalytic process that begins with the entrance of
The hydroxylase, 4-hydroxybenzoate 3-monooxygenase, proceeds through a catalytic process that begins with the entrance of NADPH
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NAD ...
and 4-hydroxybenzoate (the native substrate) into the active site of the enzyme. This results in formation of an enzyme-flavin-substrate-NADPH complex, after which the flavin cofactor, FAD
A fad or trend is any form of collective behavior that develops within a culture, a generation or social group in which a group of people enthusiastically follow an impulse for a short period.
Fads are objects or behaviors that achieve short- ...
, is reduced by NADPH. NADP+ is lost and O2 enters into the complex, followed by oxidation of the flavin to form a hydroperoxide, which acts as the hydroxide transfer reagent. It is important to note that while the group transferred is referred to as hydroxide it is formally an OH+ group. This hydroxide is transferred to the substrate from the hydroperoxide flavin, flavin-C4a-hydroperoxide, via an electrophilic aromatic substitution-type reaction. Finally, the product exits from the complex and the hydroxy-flavin is dehydrated, regenerating FAD and allowing the process to repeat.
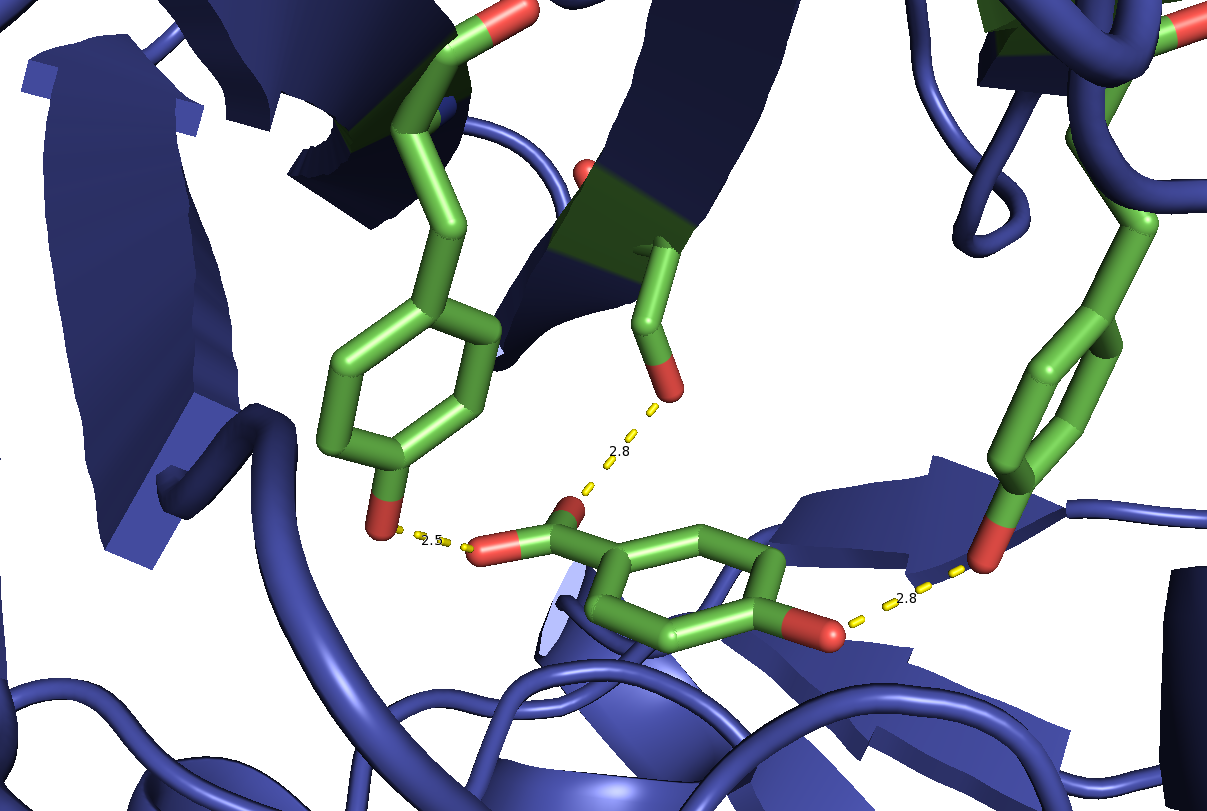
The substrate binds in the active site of the enzyme via non-covalent interactions with proximal amino acid side chains. Specifically, the hydroxyl groups of tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Gr ...
201 and 222, in addition to the hydroxyl group of serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form un ...
212, interact with the carboxylate and hydroxyl group on the substrate. This allows for proper orientation within the active site in order to achieve reactivity.
Once the substrate is bound, the flavin shifts from an “open” to a “closed” conformation. This shields the active site and substrate from solvent, preventing the premature breakdown of the flavin hydroperoxide. Binding of both NADPH and substrate shifts the enzyme to an “out” conformation. This occurs through an intricate proton network within the enzyme that allows for deprotonation of the phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it req ...
and a subsequent dynamic shift of the enzyme. The “out” conformation aligns the isoalloxazine ring of the flavin so that it can be reduced rapidly by NADPH. Following the reduction, NADP+ is released from the enzyme.
Reduction of the flavin generates a negatively charged species, FADH−, which is attracted to the positively charged active site. This attraction shifts the flavin back to the “closed” conformation, isolating it from the solvent environment. This isolation provides an optimal environment and position for O2 to hydroxylate the substrate. The oxygen binds to FADH− via a single electron transfer, which is the rate-limiting step of the reaction. This forms an FAD radical and flavin hydroperoxide. Reaction between these generates C4a-peroxyflavin, which is quickly protonated to form flavin-C4a-hydroperoxide. Tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydr ...
ization leads to the formation of 3,4-dihydoxybenzoate. The final step in the mechanism is dissociation of the product and water from FAD, causing the flavin to return to the open conformation.
References
Further reading
* * * * * * {{Portal bar, Biology, border=no EC 1.14.13 NADPH-dependent enzymes Flavoproteins Enzymes of known structure