2-Methylundecanal on:
[Wikipedia]
[Google]
[Amazon]
2-Methylundecanal is an
/ref> At high dilution it has a flavor similar to honey and nuts. It is a colorless or pale yellow liquid that is soluble in organic
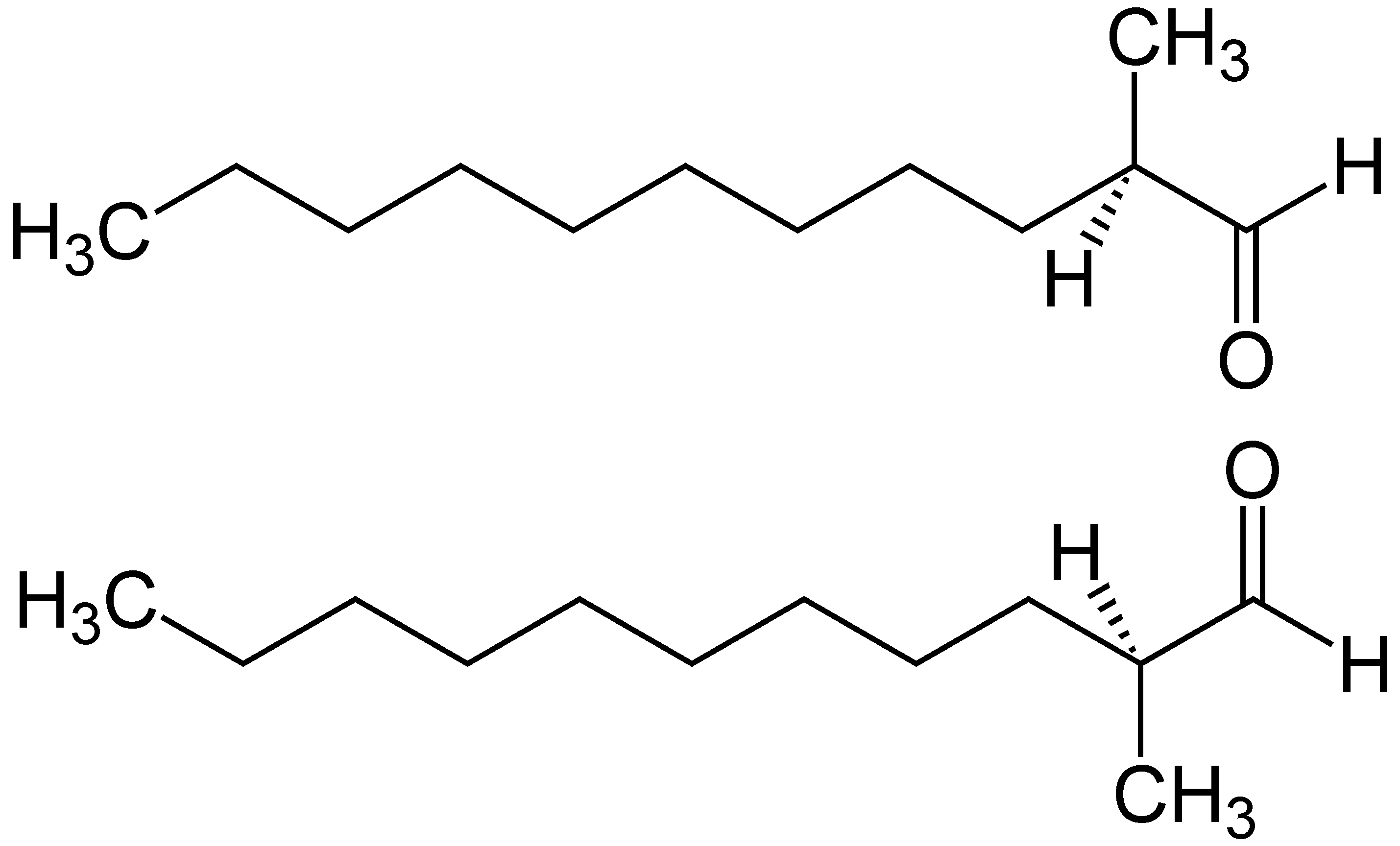 The enantiomers can be synthesized with high enantiomeric purity using the SAMP/RAMP hydrazone method. This process involves starting with simple achiral aldehydes and converting them to their SAMP hydrazones then obtaining the corresponding chiral hydrazones using RAMP as a chiral auxiliary. The chiral hydrazones are then metalated with
The enantiomers can be synthesized with high enantiomeric purity using the SAMP/RAMP hydrazone method. This process involves starting with simple achiral aldehydes and converting them to their SAMP hydrazones then obtaining the corresponding chiral hydrazones using RAMP as a chiral auxiliary. The chiral hydrazones are then metalated with
Molecule of the Month: Chanel No 5 and 2-methylundecanal
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. T ...
that is found naturally in kumquat
Kumquats (; zh, 金桔), or cumquats in Australian English, are a group of small fruit-bearing trees in the flowering plant family Rutaceae. Their taxonomy is disputed. They were previously classified as forming the now-historical genus ''For ...
peel oil. This compound smells herbaceous, orange
Orange most often refers to:
*Orange (fruit), the fruit of the tree species '' Citrus'' × ''sinensis''
** Orange blossom, its fragrant flower
*Orange (colour), from the color of an orange, occurs between red and yellow in the visible spectrum
* ...
, and ambergris
Ambergris ( or , la, ambra grisea, fro, ambre gris), ''ambergrease'', or grey amber is a solid, waxy, flammable substance of a dull grey or blackish colour produced in the digestive system of sperm whales. Freshly produced ambergris has a mari ...
-like.Molecule of the Month: Chanel No 5 and 2-methylundecanal/ref> At high dilution it has a flavor similar to honey and nuts. It is a colorless or pale yellow liquid that is soluble in organic
solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s such as ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be ...
and ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
. It is used as a fragrance component in soaps, detergents, and perfumes.
Preparation
The first synthesis of 2-methylundecanal was recorded by Georges Darzens in 1904 from methyl nonyl ketone andethyl chloroacetate
Ethyl chloroacetate is a chemical compound used primarily in the chemical industry. It is used as a solvent for organic synthesis and as an intermediate in the production of pesticides (such as sodium fluoroacetate
Sodium fluoroacetate is an or ...
. This method of synthesis can be used to produce a variety of aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s and became known as the Darzens reaction
The Darzens reaction (also known as the Darzens condensation or glycidic ester condensation) is the chemical reaction of a ketone or aldehyde with an α- haloester in the presence of a base to form an α,β-epoxy ester, also called a "glycidic es ...
and is still used today. 2-Methylundecanal is synthesized in industry by two main routes. The first route, like Darzens, involves converting methyl nonyl ketone to its glycidate by allowing it to react with alkyl chloroacetate. The glycidate then undergoes saponification
Saponification is a process of converting esters into soaps and alcohols by the action of aqueous alkali (for example, aqueous sodium hydroxide solutions). Soaps are salts of fatty acids, which in turn are carboxylic acids with long carbon chains. ...
followed by decarboxylation.
:CH3(CH2)8C(O)CH3 + ClCH2CO2CH3 → CH3(CH2)8CH(CH3)OCH(CO2R) + HCl
:CH3(CH2)8CCH3OCCO2CH3 + H2O → CH3(CH2)8CH(CH3)CHO + CO2 + ROH
The second method for the synthesis of 2-methylundecanal begins with the conversion of undecanal to 2-methyleneundecanal by allowing it to react with formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
in the presence of base. The 2-methyleneundecanal is then hydrogenated
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic ...
to give 2-methylundecanal. The required undecanal is generated from 1-decene by hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon d ...
. The resulting solution is over 50% 2-methyleneundecanal. The double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
of this compound is hydrogenated and the resulting 2-methylundecanal is separated from the by-products using fractional distillation.
:CH3(CH2)7CH2=CH2 + H2 + CO → CH3(CH2)10CHO
:CH3(CH2)10CHO + HCHO → CH3(CH2)8C(CH2)CHO + H2O
:CH3(CH2)8C(CH2)CHO + H2 → CH3(CH2)8CH(CH3)CHO
Chirality
2-Methylundecanal contains oneasymmetric carbon An asymmetric carbon atom (chiral carbon) is a carbon atom that is attached to four different types of atoms or groups of atoms. Le Bel-van't Hoff rule states that the number of stereoisomers of an organic compound is 2n, where n represents the num ...
atom.
 The enantiomers can be synthesized with high enantiomeric purity using the SAMP/RAMP hydrazone method. This process involves starting with simple achiral aldehydes and converting them to their SAMP hydrazones then obtaining the corresponding chiral hydrazones using RAMP as a chiral auxiliary. The chiral hydrazones are then metalated with
The enantiomers can be synthesized with high enantiomeric purity using the SAMP/RAMP hydrazone method. This process involves starting with simple achiral aldehydes and converting them to their SAMP hydrazones then obtaining the corresponding chiral hydrazones using RAMP as a chiral auxiliary. The chiral hydrazones are then metalated with lithium diisopropylamide
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula . It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature ...
(LDA) and alkylated
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
with a slight excess of dimethyl sulfate. Testing of the enantiomers by a professional perfumer indicated only a slight difference in odor quality and intensity.
Applications
2-Methylundecanal is used widely as afragrance
An aroma compound, also known as an odorant, aroma, fragrance or flavoring, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficiently ...
element in soap
Soap is a salt of a fatty acid used in a variety of cleansing and lubricating products. In a domestic setting, soaps are surfactants usually used for washing, bathing, and other types of housekeeping. In industrial settings, soaps are use ...
s and detergents as well as in the perfume industry to give conifer notes, fir in particular, but is also used in fantasy compositions. This aldehyde was one of the first synthetics to be used in a prestigious perfume, namely Chanel No. 5
Chanel No. 5 was the first perfume launched by French couturier Gabrielle "Coco" Chanel in 1921. The scent formula for the fragrance was compounded by French-Russian chemist and perfumer Ernest Beaux. The design of its bottle has been an impo ...
.
References
External links
Molecule of the Month: Chanel No 5 and 2-methylundecanal
Further reading
* Burdock, George A., Fenorali, Giovanni. Fenorali’s Handbook of Flavor Ingredients, 5th ed.; CRC Press: Boca Raton, 2004. * Ullmann’s Encyclopedia of Industrial Chemistry 7th Ed: Fragrances and Flavors, John Wiley & Sons Inc, Hoboken 2009. * CRC Handbook of Chemistry and Physics. 89th ed. nline2008-2009. * Darzens, Georges; Comptes Rendus Hebdomadaires des séances de l’Académie des Sciences. 1904, 139, 1214-1217. * Dieter Enders; Hubert Dyker, Synthesis and Properties of Enantiomers of the Two Artificial Fragrances Lilial and Methylundecanal. Institut für Organische Chemie der Rheinisch-Westfälischen Technischen Hochschule. 1990, 1107–1110, http://www3.interscience.wiley.com/journal/112355592/abstract?CRETRY=1&SRETRY=0. . * Ramsden, E. N. A-Level Chemistry. 4th ed. Nelson Thornes: UK, 2000. {{DEFAULTSORT:Methylundecanal, 2- Fatty aldehydes Perfumes Alkanals