1,1-bis(diphenylphosphino)methane on:
[Wikipedia]
[Google]
[Amazon]
1,1-Bis(diphenylphosphino)methane (dppm), is an
organophosphorus compound
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective in ...
with the formula CH2(PPh2)2. Dppm, a white, crystalline powder, is used in inorganic and organometallic chemistry as a ligand. It is more specifically a chelating ligand
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
because it is a ligand that can bond to metals with two phosphorus donor atoms. The natural bite angle
In coordination chemistry the bite angle is the ligand–metal–ligand bond angle of coordination complex containing a bidentate ligand. This geometric parameter is used to classify chelating ligands, including those in organometallic complexes ...
is 73°.
Synthesis and reactivity
1,1-Bis(diphenylphosphino)methane was first prepared by the reaction ofsodium diphenylphosphide
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable is ...
(Ph2PNa) with dichloromethane:
:Ph3P + 2 Na → Ph2PNa + NaPh
:2NaPPh2 + CH2Cl2 → Ph2PCH2PPh2 + 2 NaCl
The methylene group (CH2) in dppm (and especially its complexes) is mildly acidic. The ligand can be oxidized to give the corresponding oxides and sulfides CH2 (E)Ph2sub>2 (E = O, S). The methylene group is even more acidic in these derivatives.
Coordination chemistry
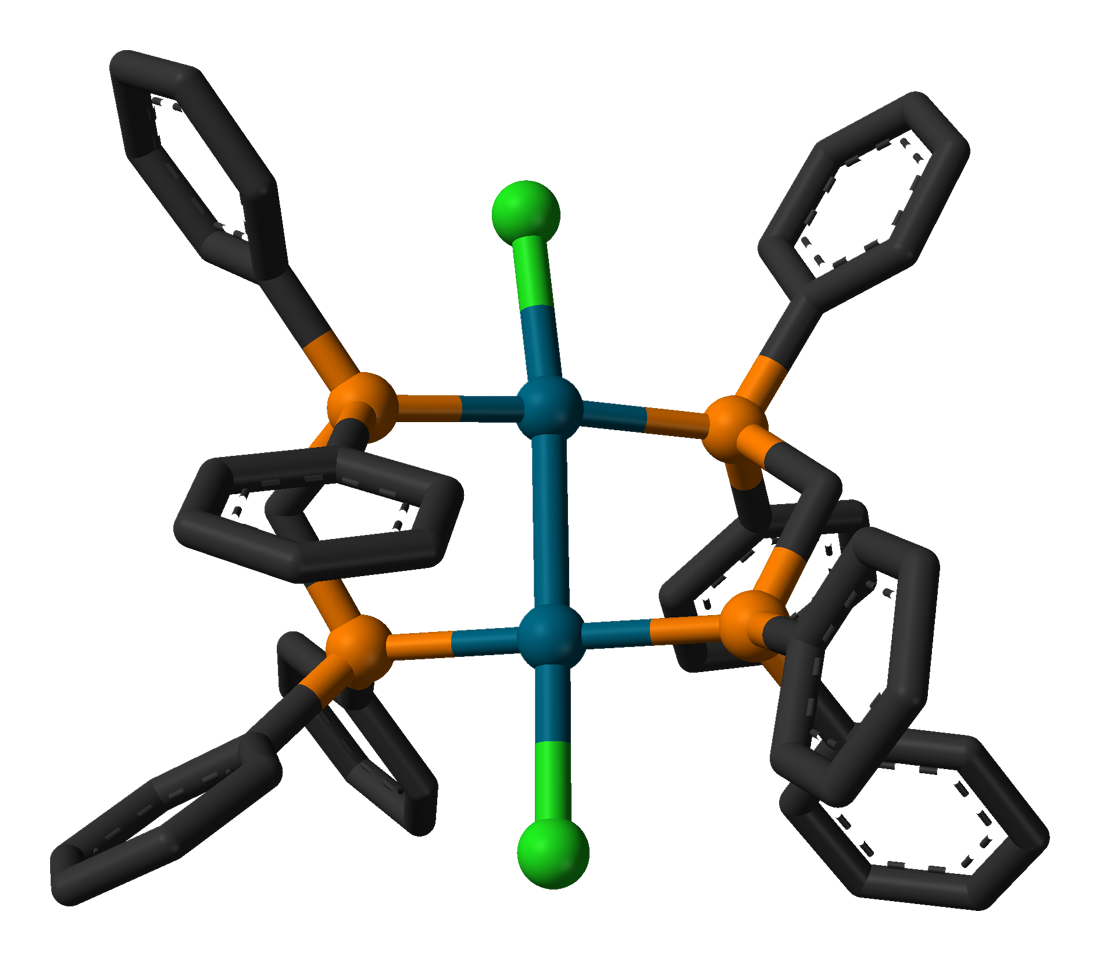
As a chelating ligand, 1,1-bis(diphenylphosphino)methane forms a four-membered ring with the constituents MP2C. The ligand promotes the formation of bimetallic complexes that feature five-membered M2P2C rings. In this way, dppm promotes the formation of bimetallic complexes. One such example is the dipalladium chloride, Pd2Cl2(dppm)2. In this complex, the oxidation state for the Pd centres are I. Bis(diphenylphosphino)methane gives rise to a family of coordination compounds known asA-frame complex
In organometallic chemistry, A-frame complexes are coordination compounds that contain two bridging bidentate ligands and a single atom bridge. They have the formula , where ''bd'' is a bidentate ligand like dppm, and X and L are a wide variet ...
es.

References
{{reflist Diphosphines Phenyl compounds