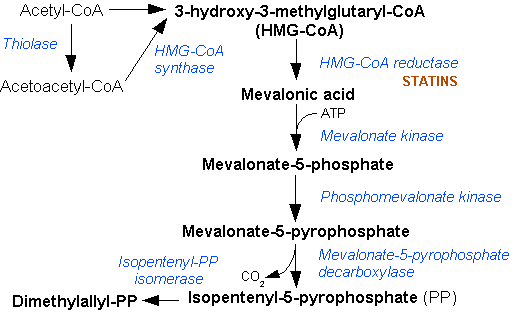
|
β-sitosterol
β-sitosterol (beta-sitosterol) is one of several phytosterols (plant sterols) with chemical structures similar to that of cholesterol. It is a white, waxy powder with a characteristic odor, and is one of the components of the food additive E499. Phytosterols are hydrophobic and soluble in alcohols. Natural occurrences and food β-sitosterol is widely distributed in the plant kingdom. It is found in vegetable oil, nuts, avocados, and derived prepared foods such as salad dressings. Human research β-sitosterol is being studied for its potential to reduce benign prostatic hyperplasia (BPH) and blood cholesterol levels. Genetic disorder While plant sterols are usually beneficial, there is a rare autosomal recessive genetic disorder phytosterolemia which causes over-absorption of phytosterols. Precursor of anabolic steroid boldenone Being a steroid, β-sitosterol is a precursor of anabolic steroid boldenone. Boldenone undecylenate is commonly used in veterinary medicine to i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytosterol
Phytosterols are phytosteroids, similar to cholesterol, that serve as structural components of biological membranes of plants. They encompass plant sterols and stanols. More than 250 sterols and related compounds have been identified. Free phytosterols extracted from oils are insoluble in water, relatively insoluble in oil, and soluble in alcohols. Phytosterol-enriched foods and dietary supplements have been marketed for decades. Despite well-documented LDL cholesterol-lowering effects from long-term consumption of phytosterols, there is insufficient evidence for an effect on cardiovascular diseases, fasting blood sugar, glycated hemoglobin, or overall mortality rate. Structure They have a fused polycyclic structure and vary in carbon side chains and / or presence or absence of a double bond (saturation). They are divided into 4,4-dimethyl phytosterols, 4-monomethyl phytosterols, and 4-desmethyl phytosterols based on the location of methyl groups at the carbon-4 positi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E499
Stigmasterol-rich plant sterols is a food additive. It is a mixture derived from soybeans that consists of the plant sterols stigmasterol, β-sitosterol, campesterol, and brassicasterol, with stigmasterol representing >85% of the mixture. Its E number E numbers ("E" stands for "Europe") are codes for substances used as food additives, including those found naturally in many foods such as vitamin C, for use within the European Union (EU) and European Free Trade Association (EFTA). Commonly ... is E499 and it is used as a stabiliser in ready-to-freeze alcoholic cocktails. References {{Chem-stub E-number additives ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydroepiandrosterone
Dehydroepiandrosterone (DHEA), also known as androstenolone, is an endogenous steroid hormone precursor. It is one of the most abundant circulating steroids in humans. DHEA is produced in the adrenal glands, the gonads, and the brain. It functions as a metabolic intermediate in the biosynthesis of the androgen and estrogen sex steroids both in the gonads and in various other tissues. However, DHEA also has a variety of potential biological effects in its own right, binding to an array of nuclear and cell surface receptors, and acting as a neurosteroid and modulator of neurotrophic factor receptors. In the United States, DHEA is sold as an over-the-counter supplement, and medication called prasterone. Biological function As an androgen DHEA and other adrenal androgens such as androstenedione, although relatively weak androgens, are responsible for the androgenic effects of adrenarche, such as early pubic and axillary hair growth, adult-type body odor, increased oiliness o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5α-Reductase Inhibitors
5α-Reductases, also known as 3-oxo-5α-steroid 4-dehydrogenases, are enzymes involved in steroid metabolism. They participate in three metabolic pathways: bile acid biosynthesis, androgen and estrogen metabolism. There are three isozymes of 5α-reductase encoded by the genes SRD5A1, SRD5A2, and SRD5A3. 5α-Reductases catalyze the following generalized chemical reaction: :a 3-oxo-5α-steroid + acceptor a 3-oxo-Δ4-steroid + reduced acceptor Where a 3-oxo-5α-steroid and acceptor are substrates, and a corresponding 3-oxo-Δ4-steroid and the reduced acceptor are products. An instance of this generalized reaction that 5α-reductase type 2 catalyzes is: :dihydrotestosterone + NADP+ \rightleftharpoons testosterone + NADPH + H+ where dihydrotestosterone is the 3-oxo-5α-steroid, NADP+ is the acceptor and testosterone is the 3-oxo-Δ4-steroid and NADPH the reduced acceptor. Production and activity The enzyme is produced in many tissues in both males and females, in the repro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bitter Melon
''Momordica charantia'' (commonly called bitter melon; Goya; bitter apple; bitter gourd; bitter squash; balsam-pear; with many more names listed below) is a tropical and subtropical vine of the family Cucurbitaceae, widely grown in Asia, Africa, and the Caribbean for its edible fruit. Its many varieties differ substantially in the shape and bitterness of the fruit. Bitter melon originated in Africa where it was a dry-season staple food of ǃKung hunter-gatherers. Wild or semi-domesticated variants spread across Asia in prehistory, and it was likely fully domesticated in Southeast Asia. It is widely used in the cuisines of East Asia, South Asia, and Southeast Asia. Alternative names Bitter melon has many names in other languages, which have sometimes entered English as loanwords. Following are a few: Description This herbaceous, tendril-bearing vine grows up to in length. It bears simple, alternate leaves across, with three to seven deeply separated lob ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charantin
Charantin is a chemical substance obtained from the Asian bitter melon (''Momordica charantia''), reputed to be responsible for the hypoglycaemic properties of those plants. It was identified by Lolitkar and Rao in 1960. It was also found in the similar African species '' M. foetida'', by A. Olaniyi in 1975, under the name foetidin. Charantin is actually a 1:1 mixture of two steroidal saponins, β-sitosteryl glucoside () and 5,22-stigmasteryl glucoside (). It is a whitish crystalline substance, neutral and tasteless, melting at 266–268 °C. It is sparingly soluble in water or other highly polar solvents, as well as in apolar solvents like hexane, but is soluble in ether, ethanol and methanol, and can be efficiently extracted from the plant by pressurized ethanol or acetone at 100 °C. The name charantin has also been used by A. Parkash and other for a different compound, a peptide with molecular mass 9.7 kDa, also isolated from bitter melon seeds. See also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triterpene
Triterpenes are a class of chemical compounds composed of three terpene units with the molecular formula C30H48; they may also be thought of as consisting of six isoprene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids. Structures Triterpenes exist in a great variety of structures. Nearly 200 different skeletons have been identified. These skeletons may be broadly divided according to the number of rings present. In general pentacyclic structures (5 rings) tend to dominate. Squalene is biosynthesized through the head-to-head condensation of two farnesyl pyrophosphate units. This coupling converts a pair of C15 components into a C30 product. Squalene serves as precursor for the formation of many triterpenoids, including bacterial hopanoids and eukaryotic sterols. Triterpenoids By definition triterpenoids are triterpenes that possess heteroatoms, usually oxygen. The terms ''triterpene'' and ''triterpenoi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Squalene
Squalene is an organic compound. It is a triterpenoid with the formula C30H50. It is a colourless oil, although impure samples appear yellow. It was originally obtained from shark liver oil (hence its name, as ''Squalus'' is a genus of sharks). All plants and animals produce squalene as a biochemical intermediate to sterol biosynthesis. An estimated 12% of bodily squalene in humans is found in sebum. Squalene has a role in topical skin lubrication and protection. Squalene is a precursor for synthesis of all plant and animal sterols, including cholesterol and steroid hormones in the human body. Squalene is an important ingredient in some vaccine adjuvants: The Novartis and GlaxoSmithKline adjuvants are called MF59 and AS03, respectively. Role in steroid synthesis Squalene is the biochemical precursor to steroids. The squalene conversion begins with oxidation (via squalene monooxygenase) of one of its terminal double bonds, resulting in 2,3-oxidosqualene. It then undergoes an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Farnesyl Diphosphate
Farnesyl pyrophosphate (FPP), also known as farnesyl diphosphate (FDP), is an intermediate in the biosynthesis of terpenes and terpenoids such as sterols and carotenoids. It is also used in the synthesis of CoQ (part of the electron transport chain), as well as dehydrodolichol diphosphate (a precursor of dolichol, which transports proteins to the ER lumen for ''N''-glycosylation). Biosynthesis Farnesyl pyrophosphate synthase (a prenyl transferase) catalyzes sequential condensation reactions of dimethylallyl pyrophosphate Dimethylallyl pyrophosphate (DMAPP; or alternatively, dimethylallyl diphosphate (DMADP); also isoprenyl pyrophosphate) is an isoprenoid precursor. It is a product of both the mevalonate pathway and the MEP pathway of isoprenoid precursor biosynt ... with 2 units of 3-isopentenyl pyrophosphate to form farnesyl pyrophosphate, as is shown in the following two steps: * Dimethylallyl pyrophosphate reacts with 3-isopentenyl pyrophosphate to form geranyl pyrop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylallyl Diphosphate
Dimethylallyl pyrophosphate (DMAPP; or alternatively, dimethylallyl diphosphate (DMADP); also isoprenyl pyrophosphate) is an isoprenoid precursor. It is a product of both the mevalonate pathway and the MEP pathway of isoprenoid precursor biosynthesis. It is an isomer of isopentenyl pyrophosphate (IPP) and exists in virtually all life forms. The enzyme isopentenyl pyrophosphate isomerase catalyzes isomerization between DMAPP and IPP. In the mevalonate pathway DMAPP is synthesised from mevalonic acid. In contrast, DMAPP is synthesised from HMBPP in the MEP pathway. At present, it is believed that there is crossover between the two pathways in organisms that use both pathways to create terpene Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes ...s and terpenoids, such as in plants ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopentenyl Diphosphate
Isopentenyl pyrophosphate (IPP, isopentenyl diphosphate, or IDP) is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway (commonly called the mevalonate pathway) and in the ''non-mevalonate'' MEP pathway of isoprenoid precursor biosynthesis. Isoprenoid precursors such as IPP, and its isomer DMAPP, are used by organisms in the biosynthesis of terpenes and terpenoids. Biosynthesis IPP is formed from acetyl-CoA via the mevalonate pathway (the "upstream" part), and then is isomerized to dimethylallyl pyrophosphate by the enzyme isopentenyl pyrophosphate isomerase. IPP can be synthesised via an alternative non-mevalonate pathway of isoprenoid precursor biosynthesis, the MEP pathway, where it is formed from (''E'')-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) by the enzyme HMB-PP reductase (LytB, IspH). The MEP pathway is present in many bacteria, apicomplexan protozoa such as malaria Malaria is a mosquito-borne infect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloartenol
Cycloartenol is an important triterpenoid of the sterol class which is found in plants. It is the starting point for the synthesis of almost all plant steroids, making them chemically distinct from the steroids of fungi and animals, which are, instead, produced from lanosterol. Synthesis The biosynthesis of cycloartenol starts from the triterpenoid squalene. It is the first precursor in the biosynthesis of other stanols and sterols, referred to as phytostanols and phytosterol Phytosterols are phytosteroids, similar to cholesterol, that serve as structural components of biological membranes of plants. They encompass plant sterols and stanols. More than 250 sterols and related compounds have been identified. Free phyt ...s in photosynthetic organisms and plants. The identities and distribution of phytostanols and phytosterols is characteristic of a plant species. References Sterols Triterpenes Cyclopropanes {{Alcohol-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
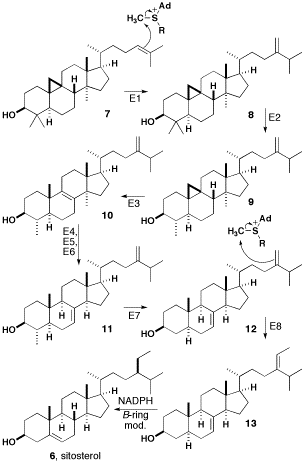

.jpg)