|
Veratrine
Veratridine is a steroidal alkaloid found in plants of the lily family, specifically the genera ''Veratrum'' and ''Schoenocaulon''. Upon absorption through the skin or mucous membranes, it acts as a neurotoxin by binding to and preventing the inactivation of voltage-gated sodium ion channels in heart, nerve, and skeletal muscle cell membranes. Veratridine increases nerve excitability and intracellular Ca2+ concentrations. Isolation Veratridine has been isolated from the seeds of ''Schoenocaulon officinale'' and from the rhizomes of ''Veratrum album.'' Like the other steroidal alkaloids found in these plants and similar ones in the Melanthiaceae family, it is present as part of a glycosidal combination, bonded to carbohydrate moieties. Early isolation methods relied on formation of the nitrate salt and then precipitation of the insoluble sulfate form. Accounts of these efforts date back to 1878, but the first true purification of veratridine is the one carried out in 1953 by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Veratrum Album
''Veratrum album'', the false helleborine, white hellebore, European white hellebore, or white veratrum ( syn. ''Veratrum lobelianum'' Bernh.) is a poisonous plant in the family Melanthiaceae. It is native to Europe and parts of western Asia (western Siberia, Turkey, Caucasus). Description ''Veratrum album'' is a tall herbaceous perennial plant with alternate, pleated leaves. The flowers are white, marked with green on the top portion of the stalk. The fruit is a small pod containing winged seeds. The stout, simple stems are tall. The plants have an estimated lifespan of several centuries and often achieve dominance in wild areas as they are unpalatable to grazing herbivores. Uses Extracts from dried rhizomes of ''Veratrum album'' were briefly used as a pesticide against the Colorado potato beetle. Research In 1890, Georg Salzberger first isolated and named the alkaloid ''protoveratrine''. Later investigation found that protoveratrine is a mixture of two closely related alkaloi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
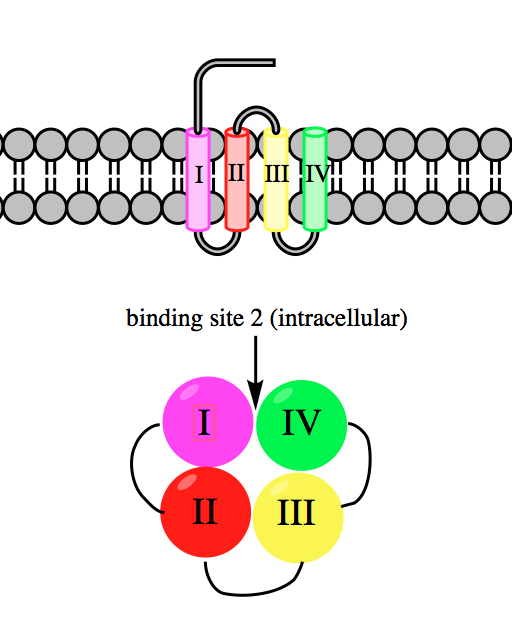
Veratridine Binding Site
Veratridine is a steroidal alkaloid found in plants of the lily family, specifically the genera ''Veratrum'' and ''Schoenocaulon''. Upon absorption through the skin or mucous membranes, it acts as a neurotoxin by binding to and preventing the inactivation of voltage-gated sodium ion channels in heart, nerve, and skeletal muscle cell membranes. Veratridine increases nerve excitability and intracellular Ca2+ concentrations. Isolation Veratridine has been isolated from the seeds of ''Schoenocaulon officinale'' and from the rhizomes of ''Veratrum album.'' Like the other steroidal alkaloids found in these plants and similar ones in the Melanthiaceae family, it is present as part of a glycosidal combination, bonded to carbohydrate moieties. Early isolation methods relied on formation of the nitrate salt and then precipitation of the insoluble sulfate form. Accounts of these efforts date back to 1878, but the first true purification of veratridine is the one carried out in 1953 by Kupch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steroid And Veratridine Ring Backbones
A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signaling molecules. Hundreds of steroids are found in plants, animals and fungi. All steroids are manufactured in cells from the sterols lanosterol (opisthokonts) or cycloartenol (plants). Lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene. The steroid core structure is typically composed of seventeen carbon atoms, bonded in four " fused" rings: three six-member cyclohexane rings (rings A, B and C in the first illustration) and one five-member cyclopentane ring (the D ring). Steroids vary by the functional groups attached to this four-ring core and by the oxidation state of the rings. Sterols are forms of steroids with a hydroxy group at position three and a skeleton derived from cholestane. ''Also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NMR Spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds. The principle of NMR usually involves three sequential steps: # The alignment (polarization) of the magnetic nuclear spins in an applied, constant magnetic field B0. # The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Batrachotoxin
Batrachotoxin (BTX) is an extremely potent cardio- and neurotoxic steroidal alkaloid found in certain species of beetles, birds, and frogs. The name is from the Greek word grc, βάτραχος, bátrachos, frog, label=none. Structurally-related chemical compounds are often referred to collectively as batrachotoxins. It is an extremely poisonous alkaloid. In certain frogs, this alkaloid is present mostly on the skin. Such frogs are among those used for poisoning darts. Batrachotoxin binds to and irreversibly opens the sodium channels of nerve cells and prevents them from closing, resulting in paralysis and death. No antidote is known. History Batrachotoxin was discovered by Fritz Märki and Bernhard Witkop, at the National Institute of Arthritis and Metabolic Diseases, National Institutes of Health, Bethesda, Maryland, U.S.A. Märki and Witkop separated the potent toxic alkaloids fraction from ''Phyllobates bicolor'' and determined its chemical properties in 1963. They isolat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hygroscopy
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they absorb so much water that they become liquid and form an aqueous solution. Etymology and pronunciation The word ''hygroscopy'' () uses combining forms of '' hygro-'' and '' -scopy''. Unlike any other ''-scopy'' word, it no longer refers to a viewing or imaging mode. It did begin that way, with the word ''hygroscope'' referring in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions .... It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. History In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi ( 865–925, Latin: Rhazes) conducted experiments with sal ammoniac (ammonium chloride) and vitriol (hydrated sulfates of various metals), which he distilled together, thus producing the gas hydrogen chloride. In doing so, al-Razi may have stumbled upon a primitive method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free Base
Free base (freebase, free-base) is the conjugate base (deprotonated) form of an amine, as opposed to its conjugate acid (protonated) form. The amine is often an alkaloid, such as nicotine, cocaine, morphine, and ephedrine, or derivatives thereof. Freebasing is a more efficient method of self-administering alkaloids via the smoking route. Properties Some alkaloids are more stable as ionic salts than as free base. The salts usually exhibit greater water solubility. Common counterions include chloride, bromide, sulfate, phosphate, nitrate, acetate, oxalate, citrate, and tartrate. Ammonium salts formed from the acid-base reaction with hydrochloric acid are known as hydrochlorides. For example, compare the free base hydroxylamine (NH2OH) with the salt hydroxylamine hydrochloride (NH3OH+ Cl−). Freebasing Cocaine hydrochloride ("powder cocaine") cannot be smoked as it decomposes at the high temperatures produced by smoking. Free base cocaine, on the other hand, has a melting poin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroform
Chloroform, or trichloromethane, is an organic compound with chemical formula, formula Carbon, CHydrogen, HChlorine, Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to PTFE. It is also a precursor to various refrigerants. It is trihalomethane. It is a powerful anesthetic, euphoriant, anxiolytic, and sedative when inhaled or ingested. Structure The molecule adopts a tetrahedral molecular geometry with C3v symmetry group, symmetry. Natural occurrence The total global flux of chloroform through the environment is approximately tonnes per year, and about 90% of emissions are natural in origin. Many kinds of seaweed produce chloroform, and fungi are believed to produce chloroform in soil. Abiotic processes are also believed to contribute to natural chloroform productions in soils although the mechanism is still unclear. Chloroform volatilizes readily from soil and surface water and undergoes degradation in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a relatively high boiling point. DMSO has the unusual property that many individuals perceive a garlic-like taste in the mouth after DMSO makes contact with their skin. In terms of chemical structure, the molecule has idealized Cs symmetry. It has a trigonal pyramidal molecular geometry consistent with other three-coordinate S(IV) compounds, with a nonbonded electron pair on the approximately tetrahedral sulfur atom. Synthesis and production Dimethyl sulfoxide was first synthesized in 1866 by the Russian scientist Alexander Zaytsev, who reported his findings in 1867. Dimethyl sulfoxide is produced industrially from dimethyl sulfide, a by-product of the Kraf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic, until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication. Production Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises. Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Ethanol is mixed with a stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |