|
Vanadium(III) Oxide
Vanadium(III) oxide is the inorganic compound with the formula V2O3. It is a black solid prepared by reduction of V2O5 with hydrogen or carbon monoxide. It is a basic oxide dissolving in acids to give solutions of vanadium (III) complexes. V2O3 has the corundum structure. It is antiferromagnetic with a critical temperature of 160 K. E.M. Page, S.A.Wass (1994),''Vanadium:Inorganic and Coordination chemistry'', Encyclopedia of Inorganic Chemistry, John Wiley & Sons, At this temperature there is an abrupt change in conductivity from metallic to insulating. This also distorts the crystal structure to a monoclinic space group: C2/c. Upon exposure to air it gradually converts into indigo-blue V2O4. In nature it occurs as the rare mineral karelianite Karelianite is an rare mineral, a natural form of vanadium(III) oxide, V2O3. In terms of chemistry it is vanadium-analogue of hematite, corundum, eskolaite, tistarite, bixbyite, avicennite Avicennite ( thallium(III) oxide) is an ox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
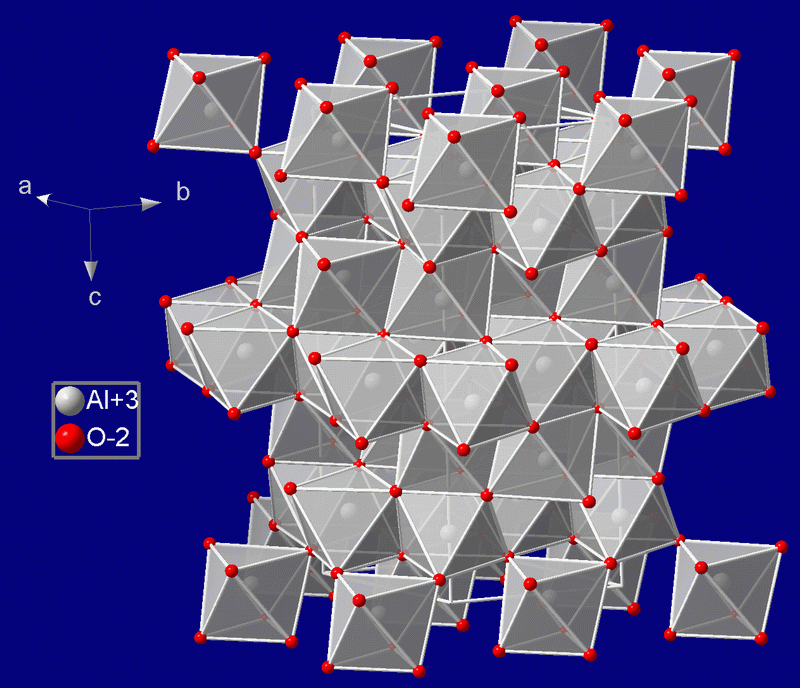
Corundum
Corundum is a crystalline form of aluminium oxide () typically containing traces of iron, titanium, vanadium and chromium. It is a rock-forming mineral. It is a naturally transparent material, but can have different colors depending on the presence of transition metal impurities in its crystalline structure. Corundum has two primary gem varieties: ruby and sapphire. Rubies are red due to the presence of chromium, and sapphires exhibit a range of colors depending on what transition metal is present. A rare type of sapphire, padparadscha sapphire, is pink-orange. The name "corundum" is derived from the Tamil- Dravidian word ''kurundam'' (ruby-sapphire) (appearing in Sanskrit as ''kuruvinda''). Because of corundum's hardness (pure corundum is defined to have 9.0 on the Mohs scale), it can scratch almost all other minerals. It is commonly used as an abrasive on sandpaper and on large tools used in machining metals, plastics, and wood. Emery, a variety of corundum with no value a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure, and was originated by W. B. Pearson. The symbol is made up of two letters followed by a number. For example: * Diamond structure, ''cF''8 * Rutile structure, ''tP''6 The two (italicised) letters specify the Bravais lattice. The lower-case letter specifies the crystal family, and the upper-case letter the centering type. The number at the end of the Pearson symbol gives the number of the atoms in the conventional unit cell.Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 IR-3.4.4, pp. 49–51; IR-11.5, pp. 241–242. |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium Pentoxide
Vanadium(V) oxide (''vanadia'') is the inorganic compound with the formula V2 O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst. The mineral form of this compound, shcherbinaite, is extremely rare, almost always found among fumaroles. A mineral trihydrate, V2O5·3H2O, is also known under the name of navajoite. Chemical properties Reduction to lower oxides Upon heating a mixture of vanadium(V) oxide and vanadium(III) oxide, comproportionation occurs to give vanadium(IV) oxide, as a deep-blue solid: :V2O5 + V2O3 → 4 VO2 The reduction can also be effected by oxalic acid, carbon monoxide, and sulfur dioxide. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds, when insufficient oxygen or heat is present to produce carbon dioxide. There are also numerous environmental and biological sources that generate and emit a significant amount of carbon monoxide. It is important in the production of many compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change. Carbon monoxide has important biological roles across phylogenetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corundum
Corundum is a crystalline form of aluminium oxide () typically containing traces of iron, titanium, vanadium and chromium. It is a rock-forming mineral. It is a naturally transparent material, but can have different colors depending on the presence of transition metal impurities in its crystalline structure. Corundum has two primary gem varieties: ruby and sapphire. Rubies are red due to the presence of chromium, and sapphires exhibit a range of colors depending on what transition metal is present. A rare type of sapphire, padparadscha sapphire, is pink-orange. The name "corundum" is derived from the Tamil- Dravidian word ''kurundam'' (ruby-sapphire) (appearing in Sanskrit as ''kuruvinda''). Because of corundum's hardness (pure corundum is defined to have 9.0 on the Mohs scale), it can scratch almost all other minerals. It is commonly used as an abrasive on sandpaper and on large tools used in machining metals, plastics, and wood. Emery, a variety of corundum with no value a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium Dioxide
Vanadium(IV) oxide or vanadium dioxide is an inorganic compound with the formula VO2. It is a dark blue solid. Vanadium(IV) dioxide is amphoteric, dissolving in non-oxidising acids to give the blue vanadyl ion, Osup>2+ and in alkali to give the brown 4O9sup>2− ion, or at high pH O4sup>4−. VO2 has a phase transition very close to room temperature (~). Electrical resistivity, opacity, etc, can change up several orders. Owing to these properties, it has been used in surface coating, sensors, and imaging. Potential applications include use in memory devices, phase-change switches, passive radiative cooling applications, such as smart windows and roofs, that cool or warm depending on temperature, aerospace communication systems and neuromorphic computing. Properties Structure At temperatures below Tc = , has a monoclinic (space group P21/c) crystal structure. Above Tc, the structure is tetragonal, like rutile . In the monoclinic phase, the V4+ ions form pairs along the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Karelianite
Karelianite is an rare mineral, a natural form of vanadium(III) oxide, V2O3. In terms of chemistry it is vanadium-analogue of hematite, corundum, eskolaite, tistarite, bixbyite, avicennite Avicennite ( thallium(III) oxide) is an oxide mineral. It was discovered around the Dzhuzumli village, Samarqand, Uzbekistan. It is named after Avicenna, a Persian doctor and polymath. See also *List of minerals *List of minerals named after peopl ..., and yttriaite-(Y). The name comes from Karelia, a region on the Finnish-Russian border. It may be associated with magnesium-rich rocks. References Oxide minerals Vanadium minerals Hematite group Trigonal minerals {{Mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium(III) Compounds
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( passivation) somewhat stabilizes the free metal against further oxidation. Spanish scientist Andrés Manuel del Río discovered compounds of vanadium in 1801 in Mexico by analyzing a new lead-bearing mineral he called "brown lead". Though he initially presumed its qualities were due to the presence of a new element, he was later erroneously convinced by French chemist Hippolyte Victor Collet-Descotils that the element was just chromium. Then in 1830, Nils Gabriel Sefström generated chlorides of vanadium, thus proving there was a new element, and named it "vanadium" after the Scandinavian goddess of beauty and fertility, Vanadís (Freyja). The name was based on the wide range of colors found in vanadium compounds. Del Rio's lead mineral was ult ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sesquioxides
A sesquioxide is an oxide of an element (or radical), where the ratio between the number of atoms of that element and the number of atoms of oxygen is 2:3. For example, aluminium oxide and phosphorus(III) oxide are sesquioxides. Many sesquioxides contain a metal in the +3 oxidation state and the oxide ion , e.g., aluminium oxide , lanthanum(III) oxide and iron(III) oxide . Sesquioxides of iron and aluminium are found in soil. The alkali metal sesquioxides are exceptions because they contain both peroxide and superoxide ions, e.g., rubidium sesquioxide is formulated . Sesquioxides of metalloids and nonmetals are exceptions too, e.g. boron trioxide , dinitrogen trioxide and phosphorus(III) oxide . Many transition metal oxides crystallize in the corundum structure type, with space group Rc. Sesquioxides of rare earth elements crystalize into one or more of three crystal structures: hexagonal (type A, space group Pm1), monoclinic (type B, space group C2/m), or body-cente ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |