|
Urochrome
Urobilin or urochrome is the chemical primarily responsible for the yellow color of urine. It is a linear pyrrole, tetrapyrrole compound that, along with the related colorless compound urobilinogen, are Heme#Degradation, degradation products of the cyclic tetrapyrrole heme. Metabolism Urobilin is generated from the degradation of heme, which is first degraded through biliverdin to bilirubin. Bilirubin is then excreted as bile, which is further degraded by microbes present in the large intestine to urobilinogen. Some of this remains in the large intestine, and its conversion to stercobilin gives feces their brown color. Some is reabsorbed into the bloodstream and then delivered to kidney. When urobilinogen is exposed to air, it is oxidized to urobilin, giving urine its yellow color. Importance Many urine tests (urinalysis) monitor the amount of urobilin in urine, as its levels can give insight on the effectiveness of urinary tract function. Normally, urine would appear as either l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biological Pigments
Biological pigments, also known simply as pigments or biochromes, are substances produced by living organisms that have a color resulting from selective color absorption. Biological pigments include plant pigments and flower pigments. Many biological structures, such as skin, eyes, feathers, fur and hair contain pigments such as melanin in specialized cells called chromatophores. In some species, pigments accrue over very long periods during an individual's lifespan. Pigment color differs from structural color in that it is the same for all viewing angles, whereas structural color is the result of selective reflection or iridescence, usually because of multilayer structures. For example, butterfly wings typically contain structural color, although many butterflies have cells that contain pigment as well. Biological pigments See conjugated systems for electron bond chemistry that causes these molecules to have pigment. * Heme/porphyrin-based: chlorophyll, bilirubin, hemocy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urine
Urine is a liquid by-product of metabolism in humans and in many other animals. Urine flows from the kidneys through the ureters to the urinary bladder. Urination results in urine being excretion, excreted from the body through the urethra. Cell (biology), Cellular metabolism generates many by-products that are rich in nitrogen and must be clearance (medicine), cleared from the Circulatory system, bloodstream, such as urea, uric acid, and creatinine. These by-products are expelled from the body during urination, which is the primary method for excreting water-soluble chemicals from the body. A urinalysis can detect nitrogenous wastes of the mammalian body. Urine plays an important role in the earth's nitrogen cycle. In balanced ecosystems, urine fertilizes the soil and thus helps plants to grow. Therefore, Reuse of excreta, urine can be used as a fertilizer. Some animals use it to territory (animal)#Scent marking, mark their territories. Historically, aged or fermented urine (kn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homogentisate
Homogentisic acid (2,5-dihydroxyphenylacetic acid) is a phenolic acid usually found in ''Arbutus unedo'' (strawberry-tree) honey. It is also present in the bacterial plant pathogen ''Xanthomonas campestris'' pv. ''phaseoli'' as well as in the yeast ''Yarrowia lipolytica'' where it is associated with the production of brown pigments. It is oxidatively dimerised to form hipposudoric acid, one of the main constituents of the 'blood sweat' of hippopotamuses. It is less commonly known as melanic acid, the name chosen by William Prout. Human pathology Accumulation of excess homogentisic acid and its oxide, named alkapton, is a result of the failure of the enzyme homogentisic acid 1,2-dioxygenase (typically due to a mutation) in the degradative pathway of tyrosine, consequently associated with alkaptonuria. Intermediate It is an intermediate in the catabolism of aromatic amino acids such as phenylalanine and tyrosine. 4-Hydroxyphenylpyruvate (produced by transamination of tyros ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shades Of Yellow
Varieties of the color yellow may differ in hue, chroma (also called saturation, intensity, or colorfulness) or lightness (or value, tone, or brightness), or in two or three of these qualities. Variations in value are also called tints and shades, a tint being a yellow or other hue mixed with white, a shade being mixed with black. A large selection of these various colors is shown below. Computer web color yellow Yellow (RGB) (X11 yellow) (color wheel yellow) The color box at right shows the most intense yellow representable in 8-bit RGB color model; yellow is a ''secondary'' color in an additive RGB space. This color is also called color wheel yellow. It is at precisely 60 degrees on the HSV color wheel, also known as the RGB color wheel ( Image of RGB color wheel:). Its complementary color is blue. Yellow (CMYK) (process yellow) (canary yellow) Process yellow (also called pigment yellow or printer's yellow), also known as canary yellow, is one of the three co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrapyrroles
Tetrapyrroles are a class of chemical compounds that contain four pyrrole or pyrrole-like rings. The pyrrole/pyrrole derivatives are linked by ( =- or -- units), in either a linear or a cyclic fashion. Pyrroles are a five-atom ring with four carbon atoms and one nitrogen atom. Tetrapyrroles are common cofactors in biochemistry and their biosynthesis and degradation feature prominently in the chemistry of life. Some tetrapyrroles form the active core of compounds with crucial biochemical roles in living systems, such as hemoglobin and chlorophyll. In these two molecules, in particular, the pyrrole macrocycle ring frames a metal atom, that forms a coordination compound with the pyrroles and plays a central role in the biochemical function of those molecules. Structure Linear tetrapyrroles (called bilanes) include: *Heme breakdown products (e.g., bilirubin, biliverdin) * Phycobilins (found in cyanobacteria) *Luciferins as found in dinoflagellates and euphausiid shrimps (krill) F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stercobilin
Stercobilin is a tetrapyrrolic bile pigment and is one end-product of heme catabolism.Boron W, Boulpaep E. Medical Physiology: A cellular and molecular approach, 2005. 984-986. Elsevier Saunders, United States. Kay IT, Weimer M, Watson CJ (1963)“The formation in vitro of stercobilin from bilirubin”Journal of Biological Chemistry. 238:1122-3. It is the chemical responsible for the brown color of human feces and was originally isolated from feces in 1932. Stercobilin (and related urobilin) can be used as a marker for biochemical identification of fecal pollution levels in rivers. Metabolism Stercobilin results from breakdown of the heme moiety of hemoglobin found in erythrocytes. Macrophages break down senescent erythrocytes and break the heme down into biliverdin, which rapidly reduces to free bilirubin. Bilirubin binds tightly to plasma proteins (especially albumin) in the blood stream and is transported to the liver, where it is conjugated with one or two glucuronic acid re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biliverdin
Biliverdin (latin for green bile) is a green tetrapyrrolic bile pigment, and is a product of heme catabolism.Boron W, Boulpaep E. Medical Physiology: a cellular and molecular approach, 2005. 984-986. Elsevier Saunders, United States. It is the pigment responsible for a greenish color sometimes seen in bruises. Metabolism Biliverdin results from the breakdown of the heme moiety of hemoglobin in erythrocytes. Macrophages break down senescent erythrocytes and break the heme down into biliverdin along with hemosiderin, in which biliverdin normally rapidly reduces to free bilirubin. Biliverdin is seen briefly in some bruises as a green color. In bruises, its breakdown into bilirubin leads to a yellowish color. Role in disease Biliverdin has been found in excess in the blood of humans suffering from hepatic diseases. Jaundice is caused by the accumulation of biliverdin or bilirubin (or both) in the circulatory system and tissues. Jaundiced skin and sclera (whites of the eyes) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bilirubin
Bilirubin (BR) (Latin for "red bile") is a red-orange compound that occurs in the normal catabolic pathway that breaks down heme in vertebrates. This catabolism is a necessary process in the body's clearance of waste products that arise from the destruction of aged or abnormal red blood cells. In the first step of bilirubin synthesis, the heme molecule is stripped from the hemoglobin molecule. Heme then passes through various processes of porphyrin catabolism, which varies according to the region of the body in which the breakdown occurs. For example, the molecules excreted in the urine differ from those in the feces. The production of biliverdin from heme is the first major step in the catabolic pathway, after which the enzyme biliverdin reductase performs the second step, producing bilirubin from biliverdin.Boron W, Boulpaep E. Medical Physiology: a cellular and molecular approach, 2005. 984–986. Elsevier Saunders, United States. Ultimately, bilirubin is broken down within ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bile Pigment
Bilins, bilanes or bile pigments are biological pigments formed in many organisms as a metabolic product of certain porphyrins. Bilin (also called bilichrome) was named as a bile pigment of mammals, but can also be found in lower vertebrates, invertebrates, as well as red algae, green plants and cyanobacteria. Bilins can range in color from red, orange, yellow or brown to blue or green. In chemical terms, bilins are linear arrangements of four pyrrole rings (tetrapyrroles). In human metabolism, bilirubin is a breakdown product of heme. A modified bilane is an intermediate in the biosynthesis and uroporphyrinogen III from porphobilinogen (PBG). Examples of bilins are found in animals (cardinal examples are bilirubin and biliverdin), and phycocyanobilin, the chromophore of the photosynthetic pigment phycocyanin, in algae and plants. In plants, bilins also serve as the photopigments of the photoreceptor protein phytochrome. An example of an invertebrate bilin is micromatabilin, whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
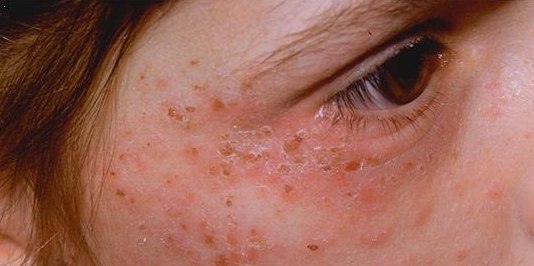
Alkaptonuria
Alkaptonuria is a rare inherited genetic disease which is caused by a mutation in the ''HGD'' gene for the enzyme homogentisate 1,2-dioxygenase (); if a person inherits an abnormal copy from both parents (it is a recessive condition), the body accumulates an intermediate substance called homogentisic acid in the blood and tissues. Homogentisic acid and its oxidized form ''alkapton'' are excreted in the urine, giving it an unusually dark color. The accumulating homogentisic acid causes damage to cartilage (ochronosis, leading to osteoarthritis) and heart valves, as well as precipitating as kidney stones and stones in other organs. Symptoms usually develop in people over 30 years old, although the dark discoloration of the urine is present from birth. Apart from treatment of the complications (such as pain relief and joint replacement for the cartilage damage), the drug nitisinone has been found to suppress homogentisic acid production, and research is ongoing as to whether it can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porphyria
Porphyria is a group of liver disorders in which substances called porphyrins build up in the body, negatively affecting the skin or nervous system. The types that affect the nervous system are also known as acute porphyria, as symptoms are rapid in onset and short in duration. Symptoms of an attack include abdominal pain, chest pain, vomiting, confusion, constipation, fever, high blood pressure, and high heart rate. The attacks usually last for days to weeks. Complications may include paralysis, low blood sodium levels, and seizures. Attacks may be triggered by alcohol, smoking, hormonal changes, fasting, stress, or certain medications. If the skin is affected, blisters or itching may occur with sunlight exposure. Most types of porphyria are inherited from one or both of a person's parents and are due to a mutation in one of the genes that make heme. They may be inherited in an autosomal dominant, autosomal recessive, or X-linked dominant manner. One type, ''porphyria c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme. Pyrroles are components of more complex macrocycles, including the porphyrinogens and products derived therefrom, including porphyrins of heme, the chlorins, bacteriochlorins, and chlorophylls. Properties Pyrrole is a colorless volatile liquid that darkens readily upon exposure to air, and is usually purified by distillation immediately before use. Pyrrole has a nutty odor. Pyrrole is a 5-membered aromatic heterocycle, like furan and thiophene. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of the heteroatom, with a dipole moment of 1.58 D. In CDCl3, it has ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |