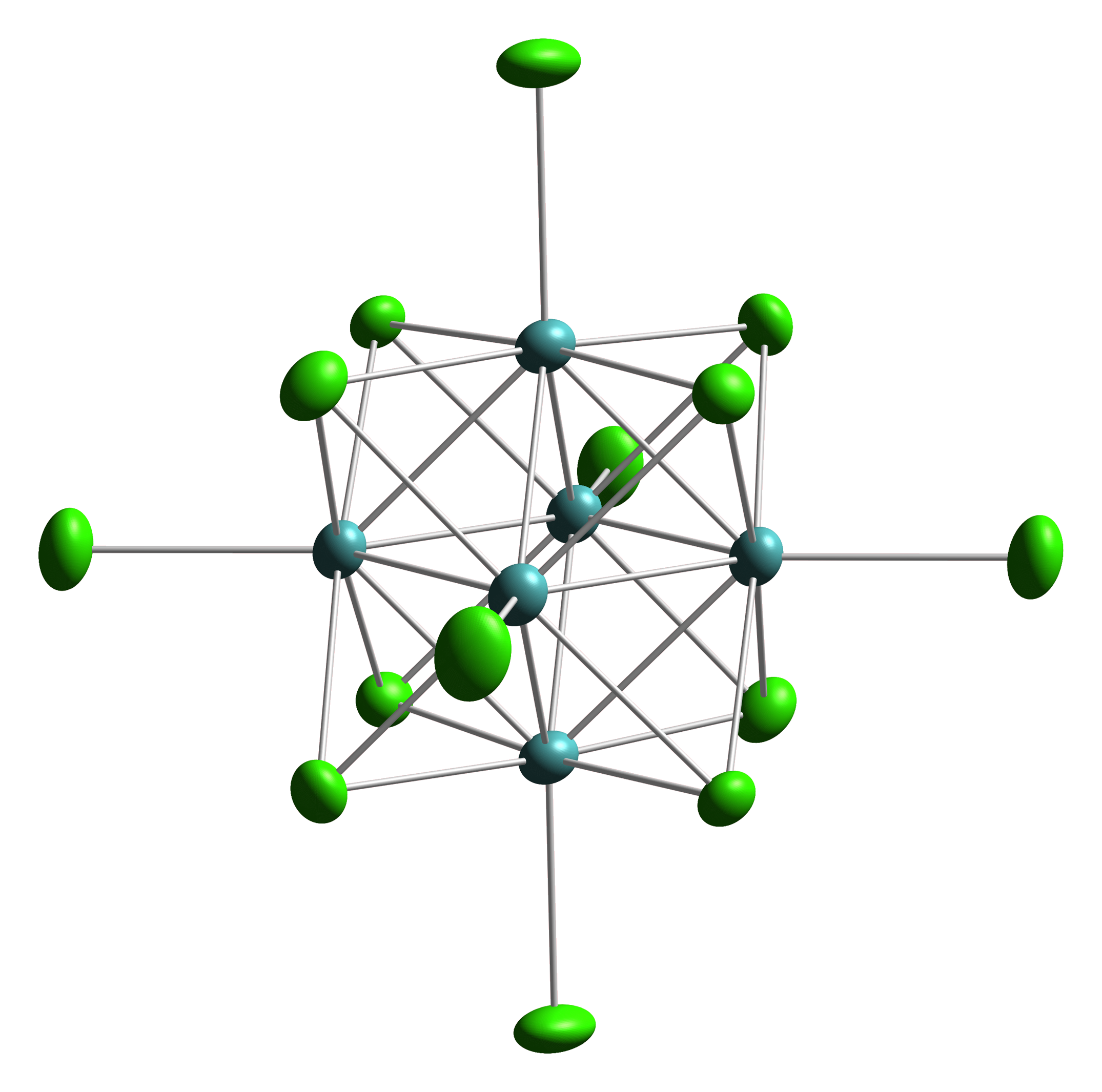
|
Tungsten(III) Chloride
Tungsten(III) chloride is the inorganic compound with the formula W6Cl18. It is a cluster compound. It is a brown solid, obtainable by chlorination of tungsten(II) chloride. Featuring twelve doubly bridging chloride ligands, the cluster adopts a structure related to the corresponding chlorides of niobium and tantalum. In contrast, W6Cl12 features eight triply bridging chlorides. A related mixed valence W(III)-W(IV) chloride is prepared by reduction of the hexachloride with bismuth Bismuth is a chemical element with the Symbol (chemistry), symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental ...: :9 WCl6 + 8 Bi → 3 W3Cl10 + 8 BiCl3 References {{Chlorides Tungsten halides Chlorides Octahedral compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedral Cluster
Octahedral clusters are inorganic or organometallic cluster compounds composed of six metals in an octahedral array.Eric J. Welch and Jeffrey R. Long ''Atomlike Building Units of Adjustable Character: Solid-State and Solution Routes to Manipulating Hexanuclear Transition Metal Chalcohalide Clusters'' in Progress in Inorganic Chemistry, Volume 54 Kenneth D. Karlin 2005''Link/ref> Many types of compounds are known, but all are synthetic. Octahedral chalcogenide and halide clusters These compounds are bound together by metal-metal bonding as well as two kinds of ligands. Ligands that span the faces or edges of the M6 core are labeled Li, for ''inner'' (innen in the original German description), and those ligands attached only to one metal are labeled outer, or La for ''ausser''. Typically, the outer ligands can be exchanged whereas the bridging ligands are more inert toward substitution. Face-capped halide clusters The premier example is of the class is Mo6Cl142−. This dianion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halogenation
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens (F2, Cl2, Br2, I2). Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride. Organic chemistry Several pathways exist for the halogenation of organic compounds, including free radical halogenation, ketone halogenation, electrophilic halogenation, and halogen addition reaction. The nature of the substrate determines the pathway. The facility of halogenation is influenced by the halogen. Fluorine and chlorine are more electrophilic and are m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten(II) Chloride
Tungsten(II) chloride is the inorganic compound with the formula W6Cl12. It is a polymeric cluster compound. The material dissolves in concentrated hydrochloric acid, forming (H3O)2 6Cl14H2O)x. Heating this salt gives yellow-brown W6Cl12. The structural chemistry resembles that observed for molybdenum(II) chloride Molybdenum dichloride describes chemical compounds with the empirical formula MoCl2. At least two forms are known, and both have attracted much attention from academic researchers because of the unexpected structures seen for these compounds and t .... Tungsten(II) chloride is prepared by reduction of the hexachloride. Bismuth is a typical reductant: :6 WCl6 + 8 Bi → W6Cl12 + 8 BiCl3 :. References {{Chlorides Tungsten halides Chlorides Octahedral compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuth
Bismuth is a chemical element with the Symbol (chemistry), symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs naturally, and its sulfide and oxide forms are important commercial ores. The free element is 86% as dense as lead. It is a brittle metal with a silvery-white color when freshly produced. Passivation (chemistry), Surface oxidation generally gives samples of the metal a somewhat rosy cast. Further oxidation under heat can give bismuth a vividly Iridescence, iridescent appearance due to thin-film interference. Bismuth is both the most Diamagnetism, diamagnetic element and one of the least Thermal conductivity, thermally conductive metals known. Bismuth was long considered the element with the highest atomic mass whose nuclei do not spontaneously decay. However, in 2003 it was discovered to be extremely weakly radioactive. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten Halides
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolated as a metal in 1783. Its important ores include scheelite and wolframite, the latter lending the element its alternate name. The free element is remarkable for its robustness, especially the fact that it has the highest melting point of all known elements barring carbon (which sublimes at normal pressure), melting at . It also has the highest boiling point, at . Its density is , comparable with that of uranium and gold, and much higher (about 1.7 times) than that of lead. Polycrystalline tungsten is an intrinsically brittle and hard material (under standard conditions, when uncombined), making it difficult to work. However, pure single-crystalline tungsten is more ductile and can be cut with a hard-steel hacksaw. Tungsten occurs in many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorides
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much larger than ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |