|
Triphenylphosphine Dichloride
Triphenylphosphine dichloride, Ph3PCl2, is a Halogenation, chlorinating agent widely used in organic chemistry. Applications include the conversion of Alcohol (chemistry), alcohols and ethers to alkyl chlorides, the cleavage of epoxides to vicinal dichlorides and the chlorination of carboxylic acids to acyl chlorides. Structure In polar solvents such as acetonitrile, Ph3PCl2 adopts an ionic phosphonium salt structure, [Ph3PCl+]Cl−, whereas in non-polar solvents like diethyl ether it exists as a non-solvated Trigonal bipyramidal molecular geometry, trigonal bipyramidal molecule. Two [Ph3PCl+] species can also adopt an unusual dinuclear ionic structure—both interacting with a Cl− via long Cl–Cl contacts. File:Chlorotriphenylphosphonium-chloride-2D.png File:Chlorotriphenylphosphonium-chloride-DCM-solvate-from-xtal-3D-balls.png File:Chlorotriphenylphosphonium-chloride-DCM-solvate-from-xtal-3D-vdW.png Synthesis Triphenylphosphine dichloride is usually prepare ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Victor Grignard
Francois Auguste Victor Grignard (6 May 1871 – 13 December 1935) was a French chemist who won the Nobel Prize for Chemistry, Nobel Prize for his discovery of the eponymously named Grignard reagent and Grignard reaction, both of which are important in the formation of carbon–carbon bonds. Biography Grignard was the son of a sail maker. His character was described as having humble and friendly attitude. After attempting to major in mathematics, Grignard failed his entrance exams before being drafted into the army in 1892. After one year of service, he went back to pursue mathematics at the University of Lyon and finally obtained his degree Licencié ès Sciences Mathématiques in 1894. In December of the same year, he transferred to chemistry and began working with Professors Philippe Barbier (1848–1922) and Louis Bouveault (1864–1909). After working with stereochemistry and enines, Grignard was not impressed with the subject matter and asked Barbier about a new directio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyl Chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example of an acyl chloride is acetyl chloride, . Acyl chlorides are the most important subset of acyl halides. Nomenclature Where the acyl chloride moiety takes priority, acyl chlorides are named by taking the name of the parent carboxylic acid, and substituting ''-yl chloride'' for ''-ic acid''. Thus: : : When other functional groups take priority, acyl chlorides are considered prefixes — ''chlorocarbonyl-'': : Properties Lacking the ability to form hydrogen bonds, acyl chlorides have lower boiling and melting points than similar carboxylic acids. For example, acetic acid boils at 118 °C, whereas acetyl chloride boils at 51 °C. Like most carbonyl compounds, infrared spectroscopy reveals a band near 1750 cm−1. The simplest s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reagents For Organic Chemistry
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a substance ''consumed'' in the course of a chemical reaction. ''Solvents'', though involved in the reaction mechanism, are usually not called reactants. Similarly, ''catalysts'' are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates. Definitions Organic chemistry In organic chemistry, the term "reagent" denotes a chemical ingredient (a compound or mixture, typically of inorganic or small organic molecules) introduced to cause the desired transformation of an organic substance. Examples include the Collins reagent, Fenton's reagent, and Grignard reagents. Analytical chemistry In analytical chemistry, a reagent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride. History Phosphorus trichloride was first prepared in 1808 by the French chemists Joseph Louis Gay-Lussac and Louis Jacques Thénard by heating calomel (Hg2Cl2) with phosphorus. Later during the same year, the English chemist Humphry Davy produced phosphorus trichloride by burning phosphorus in chlorine gas. Preparation World production exceeds one-third of a million tonnes. Phosphorus trichloride is prepared industrially by the reaction of chlorine with white phosphorus, using phosphorus trichloride as the solvent. In this continuous process PCl3 is removed as it is formed in order to avoid the formation of PCl5. :P4 + 6 Cl2 → 4 PCl3 Structure and spectroscopy It has a trig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylphosphine Oxide
Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula OP(C6H5)3, also written as Ph3PO or PPh3O (Ph = phenyl, C6H5). This colourless crystalline compound is a common but potentially useful waste product in reactions involving triphenylphosphine. It is a popular reagent to induce the crystallization, crystallizing of chemical compounds. Structure and properties Ph3PO is a tetrahedral molecule related to POCl3. The oxygen center is relatively basic. The rigidity of the backbone and the basicity of the oxygen center make this species a popular agent to crystallize otherwise difficult to crystallize molecules. This trick is applicable to molecules that have acidic hydrogen atoms, e.g. phenols. Up to now, several modifications of Ph3PO have been found: For example, a monoclinic form crystalizes in the space group ''P''21/''c'' with Z = 4 and a = 15.066(1) Å, b = 9.037(2) Å, c = 11.296(3) Å, and β = 98.47(1)°. The orthorhombic modif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stoichiometry
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions. Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products, leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of the products can be empirically determined, then the amount of the other reactants can also be calculated. This is illustrated in the image here, where the balanced equation is: : Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. This particular chemical equation is an example of complete combustion. St ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether. Preparation and structure Triphenylphosphine can be prepared in the laboratory by treatment of phosphorus trichloride with phenylmagnesium bromide or phenyllithium. The industrial synthesis involves the reaction between phosphorus trichloride, chlorobenzene, and sodium: :PCl3 + 3 PhCl + 6 Na → PPh3 + 6 NaCl Triphenylphosphine crystallizes in triclinic and monoclinic modification. In both cases, the molecule adopts a pyramidal structure with propeller-like arrangement of the three phenyl groups. Principal reactions with chalcogens, halogens, and acids Oxidation Triphenylphosphine undergoes slow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
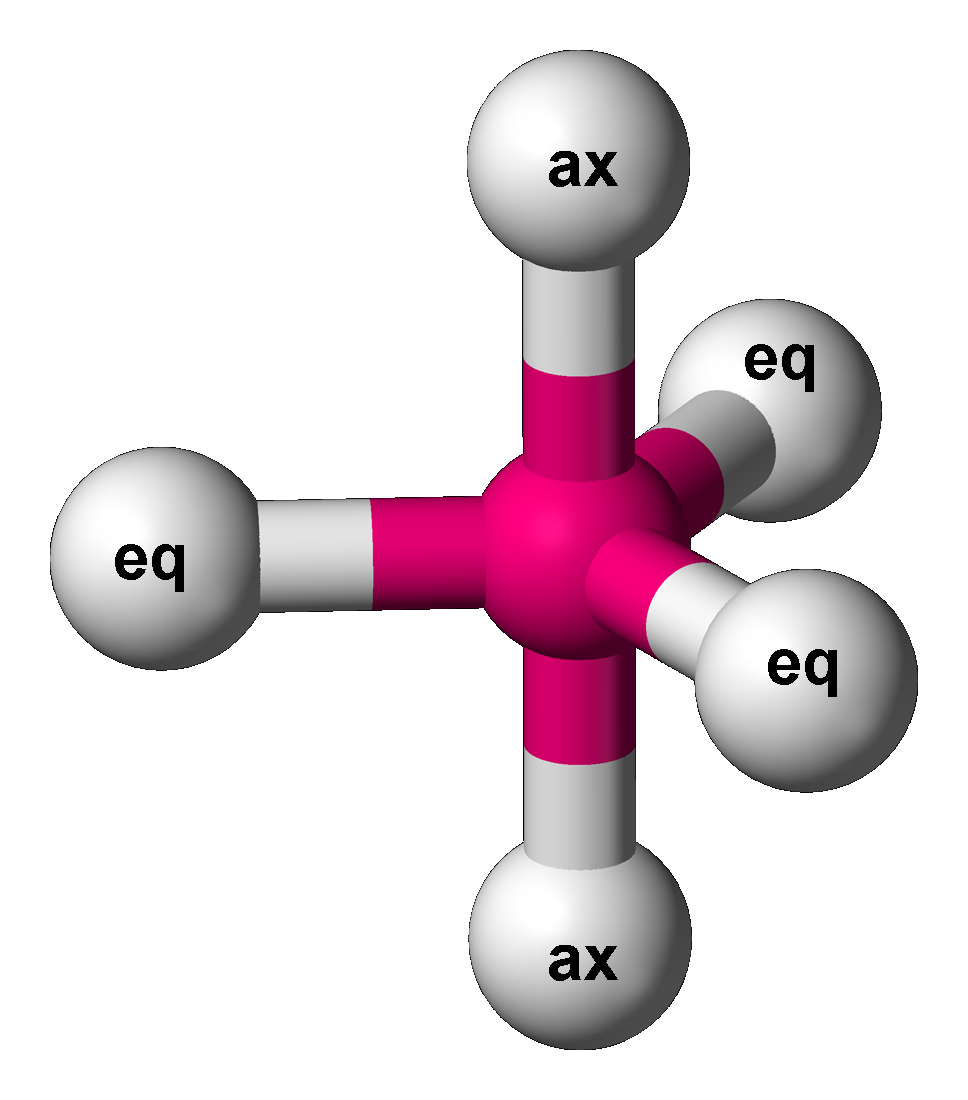
Trigonal Bipyramidal Molecular Geometry
In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid), because there is no geometrical arrangement with five terminal atoms in equivalent positions. Examples of this molecular geometry are phosphorus pentafluoride (), and phosphorus pentachloride () in the gas phase. Axial (or apical) and equatorial positions The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For phosphorus pentachloride as an example, the phosphorus atom shares a plane with three chlorine atoms at 120° angles to each other in ''equatorial'' positions, and two more chlorine atoms above and below the plane (''axial'' or ''apical'' positions). According to the VSEPR theory of molecular geometry, an axial position is more crowd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic, until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication. Production Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises. Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Ethanol is mixed with a stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Communications
''ChemComm'' (or ''Chemical Communications''), formerly known as ''Journal of the Chemical Society D: Chemical Communications'' (1969–1971), ''Journal of the Chemical Society, Chemical Communications'' (1972–1995), is a peer-reviewed scientific journal published by the Royal Society of Chemistry. It covers all aspects of chemistry. In January 2012, the journal moved to publishing 100 issues per year. The current chair of the Editorial Board is Douglas Stephan (University of Toronto, Canada), while the executive editor is Richard Kelly. Abstracting and indexing The journal is abstracted and indexed in: * Chemical Abstracts * Science Citation Index * Current Contents/Physical, Chemical & Earth Sciences * Scopus * Index Medicus/MEDLINE/PubMed According to the ''Journal Citation Reports'', the journal has a 2021 impact factor of 6.065. See also * ''New Journal of Chemistry'' * ''Chemical Society Reviews'' * ''Chemical Science'' * ''RSC Advances ''RSC Advances'' is an online ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphonium Salt
In polyatomic cations with the chemical formula (where R is a hydrogen or an alkyl, aryl, or halide group). These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions. Types of phosphonium cations Protonated phosphines The parent phosphonium is as found in the iodide salt, phosphonium iodide. Salts of the parent are rarely encountered, but this ion is an intermediate in the preparation of the industrially useful tetrakis(hydroxymethyl)phosphonium chloride: :PH3 + HCl + 4 CH2O → Many organophosphonium salts are produced by protonation of primary, secondary, and tertiary phosphines: :PR3 + H+ → The basicity of phosphines follows the usual trends, with R = alkyl being more basic than R = aryl. Tetraorganophosphonium cations The most common phosphonium compounds have four organic substituents attached to phosphorus. The quaternary phosphonium cations include tetraphenylphosphonium, (C6H5)4P+ and tetramethylphosphonium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not classed as organic). It is produced mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene. The skeleton is linear with a short distance of 1.16 Å. Acetonitrile was first prepared in 1847 by the French chemist Jean-Baptiste Dumas. Applications Acetonitrile is used mainly as a solvent in the purification of butadiene in refineries. Specifically, acetonitrile is fed into the top of a distillation column filled with hydrocarbons including butadiene, and as the acetonitrile falls down through the column, it absorbs the butadiene which is then sent from the bottom of the tower to a second separating tower. Heat is then employed in the separatin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
nickel(II)-from-xtal-3D-balls.png)