|
Transition Metal Hydride
Transition metal hydrides are chemical compounds containing a transition metal bonded to hydrogen. Most transition metals form hydride complexes and some are significant in various catalytic and synthetic reactions. The term "hydride" is used loosely: some of them are acidic (e.g., H2Fe(CO)4), whereas some others are hydridic, having H−-like character (e.g., ZnH2). Classes of metal hydrides Binary metal hydrides Many transition metals form compounds with hydrogen, called binary hydrides: binary, because these compounds contain only two elements, and hydride, because the hydrogenic ligand is assumed to have hydridic (H−-like) character. These compounds are invariably insoluble in all solvents, reflecting their polymeric structures. They often exhibit metal-like electrical conductivity. Many are nonstoichiometric compounds. Electropositive metals ( Ti, Zr, Hf, Zn) and some other metals form hydrides with the stoichiometry MH or sometimes MH2 (M = Ti, Zr, Hf, V, Zn). The b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
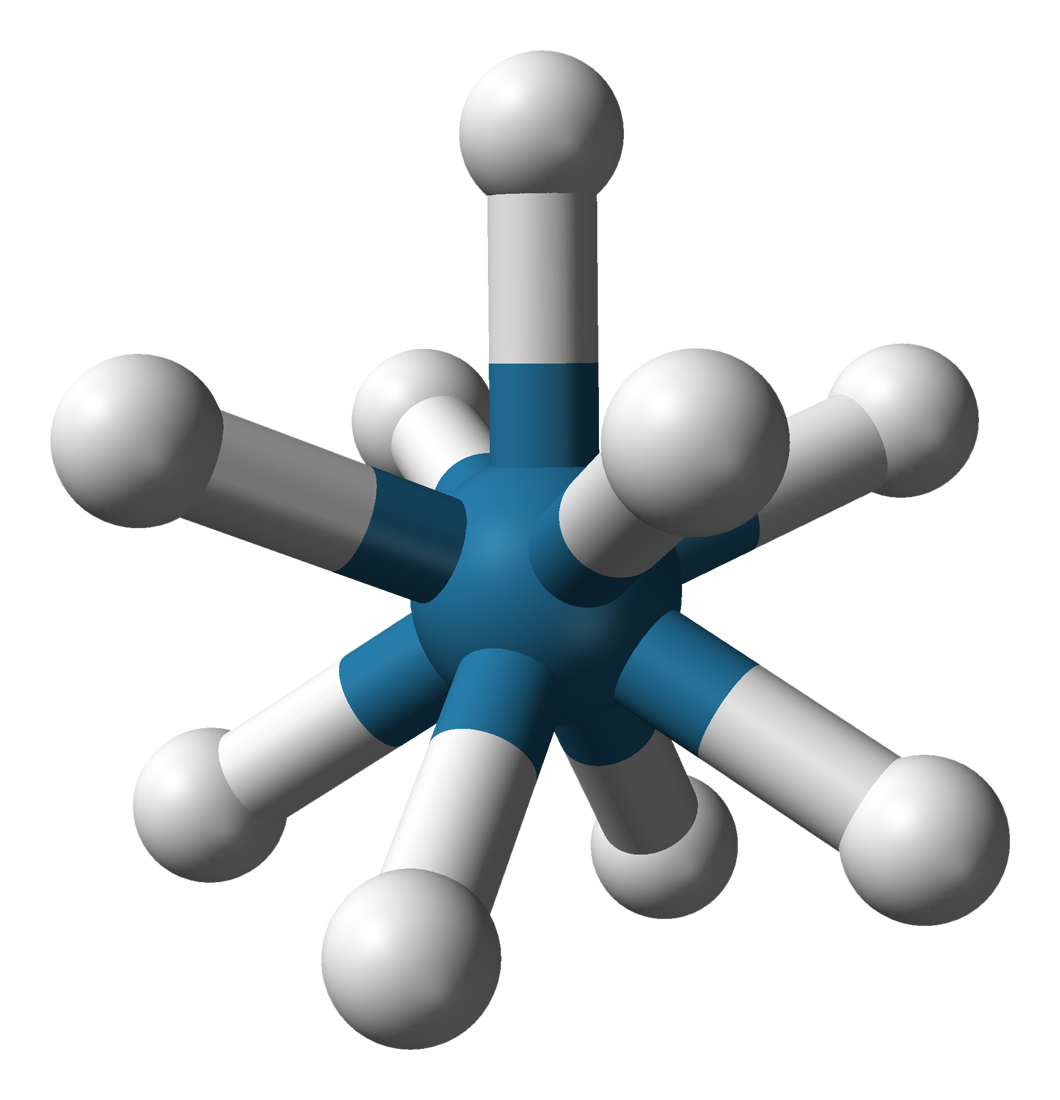
Potassium Nonahydridorhenate
Potassium nonahydridorhenate(VII) is an inorganic compound having the formula K2ReH9. This colourless salt is soluble in water but only poorly soluble in most alcohols. The anion is a rare example of a coordination complex bearing only hydride ligands. History The study of rhenium hydrides can be traced to the 1950s and included reports of the "rhenide" anion, supposedly Re−. These reports led to a series of investigations by A. P. Ginsberg and coworkers on the products from the reduction of perrhenate. The ''rhenide'' anion, Re−, was based on the product of the reduction of perrhenate salts, such as the reduction of potassium perrhenate () by potassium metal. "Potassium rhenide" was shown to exist as a tetrahydrated complex, with the postulated chemical formula . This compound exhibits strongly reducing properties, and slowly yields hydrogen gas when dissolved in water. The lithium and thallous salts were also reported. Later research, however, indicates that the "rheni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobalt Tetracarbonyl Hydride
Cobalt tetracarbonyl hydride is an organometallic compound with the formula H Co(CO)4. It is a volatile, yellow liquid that forms a colorless vapor and has an intolerable odor. The compound readily decomposes upon melt and ''in absentia'' of high CO partial pressures forms Co2(CO)8. Despite operational challenges associated with its handling, the compound has received considerable attention for its ability to function as a catalyst in hydroformylation. In this respect, HCo(CO)4 and related derivatives have received significant academic interest for their ability to mediate a variety of carbonylation (introduction of CO into inorganic compounds) reactions. Structure and properties HCo(CO)4 adopts trigonal bipyramidal structure with the equatorial CO ligands slightly bent out of the equatorial plane. The hydride ligand occupies one of the axial positions, thus the symmetry Symmetry (from grc, συμμετρία "agreement in dimensions, due proportion, arrangement") ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Walter Hieber
Walter Hieber (18 December 1895 – 29 November 1976) was an inorganic chemist, known as the father of metal carbonyl chemistry. He was born 18 December 1895 and died 29 November 1976. Hieber's father was Johannes Hieber, an influential evangelical minister and politician. Hieber was educated at Tübingen, Würzburg, and Heidelberg. In 1935 he was appointed Director of the Inorganic Chemical Institute at the Technical University in Münich. Among his numerous research findings, Hieber prepared the first metal carbonyl hydrides such as H2Fe(CO)4 and HMn(CO)5. He discovered that metal carbonyls undergo nucleophilic attack by hydroxide, the “Hieber base reaction.” He and his students discovered several metal carbonyl compounds such as Re2(CO)10 and Os3(CO)12 He pioneered the development of metal carbonyl sulfides.Hieber, W. and Scharfenberg, C., "Einwirkung organischer Schwefelverbindungen auf die Carbonyls des Eisens", Chemische Berichte, 1940, volume 73, pages 1012 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schwartz's Reagent
Schwartz's reagent is the common name for the organozirconium compound with the formula (C5H5)2ZrHCl, sometimes called zirconocene hydrochloride or zirconocene chloride hydride, and is named after Jeffrey Schwartz, a chemistry professor at Princeton University.This metallocene is used in organic synthesis for various transformations of alkenes and alkynes. Preparation The complex was first prepared by Wailes and Weigold. It can be purchased or readily prepared by reduction of zirconocene dichloride with lithium aluminium hydride: : (C5H5)2ZrCl2 + LiAlH4 → (C5H5)2ZrHCl + LiAlCl4 This reaction also affords (C5H5)2ZrH2, which is treated with methylene chloride to give Schwartz's reagent LiAl(O-t-Bu)3H can be used in place of LiAlH4. An alternative procedure that generated Schwartz's reagent from dihydride has also been reported. Moreover, it's possible to perform an ''in situ'' preparation of (C5H5)2ZrHCl from zirconocene dichloride by using LiH. This me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention: Production capacity reached 6.6×106 tons in 1995. It is important because aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in speciality chemicals, relevant to the organic synthesis of fragrances and drugs. The development of hydroformylation is one of the premier achievements of 20th-century industrial chemistry. The process entails treatment of an alkene typically with high pressures (between 10 and 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. In one variation, formaldehyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris(triphenylphosphine)rhodium Carbonyl Hydride
Carbonyl hydrido tris(triphenylphosphine)rhodium(I) arbonyl(hydrido)tris(triphenylphosphane)rhodium(I)is an organorhodium compound with the formula hH(CO)(PPh3)3(Ph = C6H5). It is a yellow, benzene-soluble solid, which is used industrially for hydroformylation. Preparation hH(CO)(PPh3)3was first prepared by the reduction of hCl(CO)(PPh3)2 e.g. with sodium tetrahydroborate, or triethylamine and hydrogen, in ethanol in the presence of excess triphenylphosphine: : hCl(CO)(PPh3)2 + NaBH4 + PPh3 → hH(CO)(PPh3)3 + NaCl + BH3 It can also be prepared from an aldehyde, rhodium trichloride and triphenylphosphine in basic alcoholic media. Structure The complex adopts a trigonal bipyramidal geometry with trans CO and hydrido ligands, resulting in ''pseudo'' -C3v symmetry. The Rh-P, Rh-C, and Rh-H distances are 2.32, 1.83, and 1.60 Å, respectively. This complex is one of a small number of stable pentacoordinate rhodium hydrides. Use in hydroformylation This precatalyst w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury Hydride
Mercury hydride may refer to: *Mercury(I) hydride Mercury(I) hydride (systematically named mercury hydride) is an inorganic compound with the chemical formula HgH. It has not yet been obtained in bulk, hence its bulk properties remain unknown. However, molecular mercury(I) hydrides with the form ... (HgH or Hg2H2), an extremely unstable gas * Mercury(II) hydride (HgH2), a volatile, relatively stable white solid {{Short pages monitor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Hydride
Cadmium hydride (systematically named cadmium dihydride) is an inorganic compound In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemist ... with the chemical formula (also written as or ). It is a solid, known only as a thermally unstable, insoluble white powder. Nomenclature The systematic name ''cadmium dihydride'', a valid IUPAC name, is constructed according to the compositional nomenclature. ''Cadmium dihydride'' is also used to refer to the related molecular compound ''dihydridocadmium'' and its oligomers. Care should be taken to avoid confusing the two compounds. ''Cadmium hydride'' is also used as a compositional IUPAC name for the compound with the chemical formula CdH. History In 1950 a research group led by Glenn D. Barbaras, synthesized cadmium hydride for the first ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

4-3D-balls.png)

