|
Toxgnostics
Toxgnostics is part of personalized medicine as it describes the guiding principles for the discovery of pharmacogenomic biomarker tests, also referred to as companion diagnostic tests, which identify if an individual patient is likely to suffer severe drug toxicity from treatment with a specific therapeutic agent. Once at-risk individuals are identified, drug toxicity can be prevented using elective dose reduction or prescription of a different medication.Church D, Kerr R, Domingo E, Rosmarin D, Palles C, Maskell K, Tomlinson I, Kerr D (2014). "'Toxgnostics': an unmet need in cancer medicine". Nat Rev Cancer (6):440-5. doi:10.1038/nrc3729. . Background The majority of toxgnostic studies have been candidate gene studies restricted to the known Absorption, Distribution, Metabolism, and Excretion genes (ADME) of drug treated patients. The PharmaADME consortium identified 32 core genes containing 184 variants within common pathways that should be included in ADME candidate gene st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Personalized Medicine
Personalized medicine, also referred to as precision medicine, is a medical model that separates people into different groups—with medical decisions, practices, interventions and/or products being tailored to the individual patient based on their predicted response or risk of disease. The terms personalized medicine, precision medicine, stratified medicine and P4 medicine are used interchangeably to describe this concept though some authors and organisations use these expressions separately to indicate particular nuances. While the tailoring of treatment to patients dates back at least to the time of Hippocrates, the term has risen in usage in recent years given the growth of new diagnostic and informatics approaches that provide understanding of the molecular basis of disease, particularly genomics. This provides a clear evidence base on which to stratify (group) related patients. Among the 14 Grand Challenges for Engineering, an initiative sponsored by National Academy of En ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacogenomic
Pharmacogenomics is the study of the role of the genome in drug response. Its name ('' pharmaco-'' + ''genomics'') reflects its combining of pharmacology and genomics. Pharmacogenomics analyzes how the genetic makeup of an individual affects their response to drugs. It deals with the influence of acquired and inherited genetic variation on drug response in patients by correlating DNA mutations (including single-nucleotide polymorphisms, copy number variations, and insertions/deletions) with pharmacokinetic (drug absorption, distribution, metabolism, and elimination), pharmacodynamic (effects mediated through a drug's biological targets), and/or immunogenic endpoints. Pharmacogenomics aims to develop rational means to optimize drug therapy, with respect to the patients' genotype, to ensure maximum efficiency with minimal adverse effects. Through the utilization of pharmacogenomics, it is hoped that pharmaceutical drug treatments can deviate from what is dubbed as the "one-d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Premarket Approval
The United States Federal Food, Drug, and Cosmetic Act (abbreviated as FFDCA, FDCA, or FD&C) is a set of laws passed by the United States Congress in 1938 giving authority to the U.S. Food and Drug Administration (FDA) to oversee the safety of food, drugs, medical devices, and cosmetics. A principal author of this law was Royal S. Copeland, a three-term U.S. senator from New York. In 1968, the Electronic Product Radiation Control provisions were added to the FD&C. Also in that year the FDA formed the Drug Efficacy Study Implementation (DESI) to incorporate into FD&C regulations the recommendations from a National Academy of Sciences investigation of effectiveness of previously marketed drugs. The act has been amended many times, most recently to add requirements about bioterrorism preparations. The introduction of this act was influenced by the death of more than 100 patients due to elixir sulfanilamide, a sulfanilamide medication where the toxic solvent diethylene glycol was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Companion Diagnostic Test
Companion may refer to: Relationships Currently * Any of several interpersonal relationships such as friend or acquaintance * A domestic partner, akin to a spouse * Sober companion, an addiction treatment coach * Companion (caregiving), a caregiver, such as a nurse assistant, paid to give a patient one-on-one attention Historically * A concubine, a long-term sexual partner not accorded the status of marriage * Lady's companion, a historic term for a genteel woman who was paid to live with a woman of rank or wealth * Companion cavalry, the elite cavalry of Alexander the Great * Foot Companion, the primary type of soldier in the army of Alexander the Great * Companions of William the Conqueror, those who took part in the Norman conquest of England * Muhammad's companions, the Sahaba, the friends who surrounded the prophet of Islam Film and television * Companion (''Doctor Who''), a character who travels with the Doctor in the TV series ''Doctor Who'' * Companion (''Firefly''), a t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Whole Genome Sequencing
Whole genome sequencing (WGS), also known as full genome sequencing, complete genome sequencing, or entire genome sequencing, is the process of determining the entirety, or nearly the entirety, of the DNA sequence of an organism's genome at a single time. This entails sequencing all of an organism's chromosomal DNA as well as DNA contained in the mitochondrial DNA, mitochondria and, for plants, in the chloroplast. Whole genome sequencing has largely been used as a research tool, but was being introduced to clinics in 2014. In the future of personalized medicine, whole genome sequence data may be an important tool to guide therapeutic intervention. The tool of DNA sequencing, gene sequencing at Single-nucleotide polymorphism, SNP level is also used to pinpoint functional variants from association studies and improve the knowledge available to researchers interested in evolutionary biology, and hence may lay the foundation for predicting disease susceptibility and drug response. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
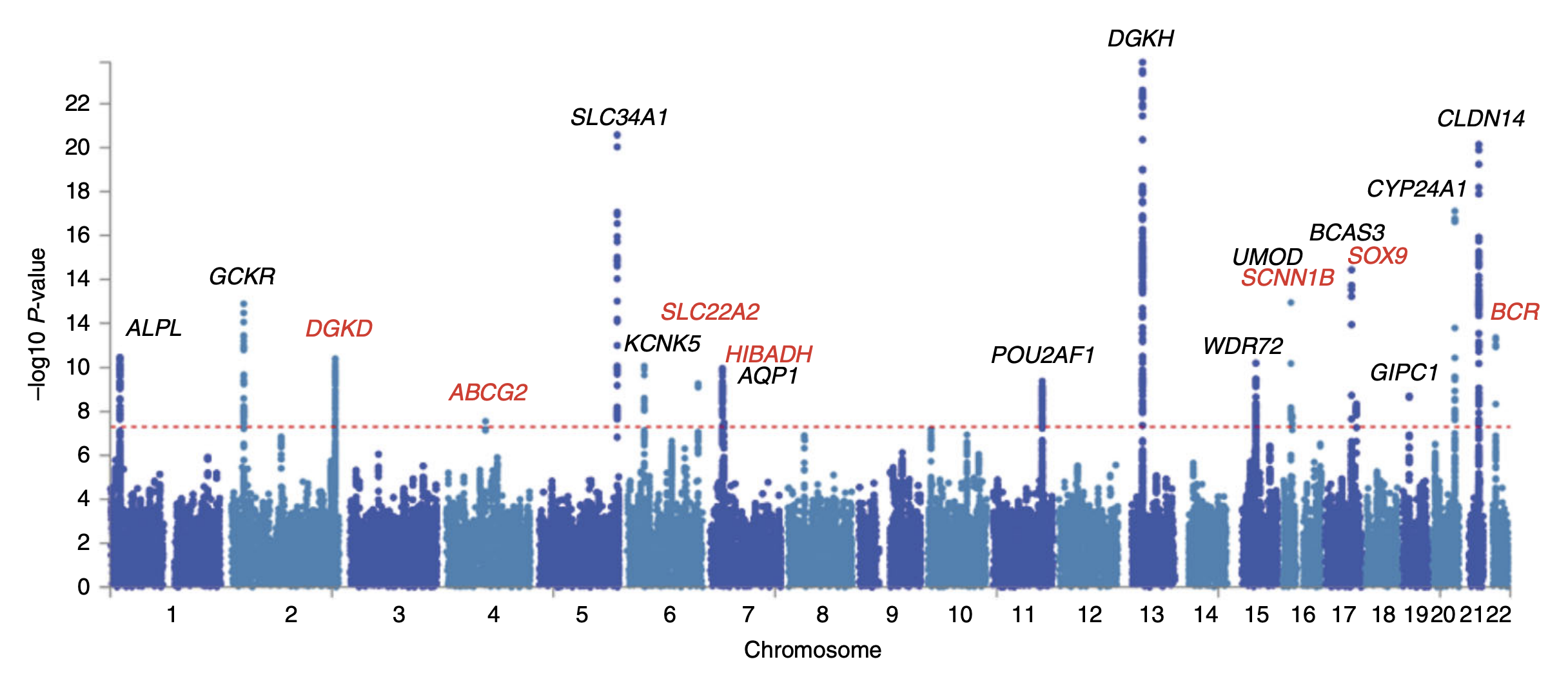
Genome-wide Association Study
In genomics, a genome-wide association study (GWA study, or GWAS), also known as whole genome association study (WGA study, or WGAS), is an observational study of a genome-wide set of Single-nucleotide polymorphism, genetic variants in different individuals to see if any variant is associated with a trait. GWA studies typically focus on associations between single-nucleotide polymorphisms (SNPs) and traits like major human diseases, but can equally be applied to any other genetic variants and any other organisms. When applied to human data, GWA studies compare the DNA of participants having varying phenotypes for a particular trait or disease. These participants may be people with a disease (cases) and similar people without the disease (controls), or they may be people with different phenotypes for a particular trait, for example blood pressure. This approach is known as phenotype-first, in which the participants are classified first by their clinical manifestation(s), as oppose ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Candidate Gene
The candidate gene approach to conducting genetic association studies focuses on associations between genetic variation within pre-specified genes of interest, and phenotypes or disease states. This is in contrast to genome-wide association studies (GWAS), which is a hypothesis-free approach that scans the entire genome for associations between common genetic variants (typically SNPs) and traits of interest. Candidate genes are most often selected for study based on ''a priori'' knowledge of the gene's biological functional impact on the trait or disease in question. The rationale behind focusing on allelic variation in specific, biologically relevant regions of the genome is that certain alleles within a gene may directly impact the function of the gene in question and lead to variation in the phenotype or disease state being investigated. This approach often uses the case-control study design to try to answer the question, "Is one allele of a candidate gene more frequently seen in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacodynamics
Pharmacodynamics (PD) is the study of the biochemical and physiologic effects of drugs (especially pharmaceutical drugs). The effects can include those manifested within animals (including humans), microorganisms, or combinations of organisms (for example, infection). Pharmacodynamics and pharmacokinetics are the main branches of pharmacology, being itself a topic of biology interested in the study of the interactions between both endogenous and exogenous chemical substances with living organisms. In particular, pharmacodynamics is the study of how a drug affects an organism, whereas pharmacokinetics is the study of how the organism affects the drug. Both together influence dosing, benefit, and adverse effects. Pharmacodynamics is sometimes abbreviated as PD and pharmacokinetics as PK, especially in combined reference (for example, when speaking of PK/PD models). Pharmacodynamics places particular emphasis on dose–response relationships, that is, the relationships between d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacokinetics
Pharmacokinetics (from Ancient Greek ''pharmakon'' "drug" and ''kinetikos'' "moving, putting in motion"; see chemical kinetics), sometimes abbreviated as PK, is a branch of pharmacology dedicated to determining the fate of substances administered to a living organism. The substances of interest include any chemical xenobiotic such as: pharmaceutical drugs, pesticides, food additives, cosmetics, etc. It attempts to analyze chemical metabolism and to discover the fate of a chemical from the moment that it is administered up to the point at which it is completely eliminated from the body. Pharmacokinetics is the study of how an organism affects a drug, whereas pharmacodynamics (PD) is the study of how the drug affects the organism. Both together influence dosing, benefit, and adverse effects, as seen in PK/PD models. Overview Pharmacokinetics describes how the body affects a specific xenobiotic/chemical after administration through the mechanisms of absorption and distribution, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)