|
Tin(II) Hydroxide
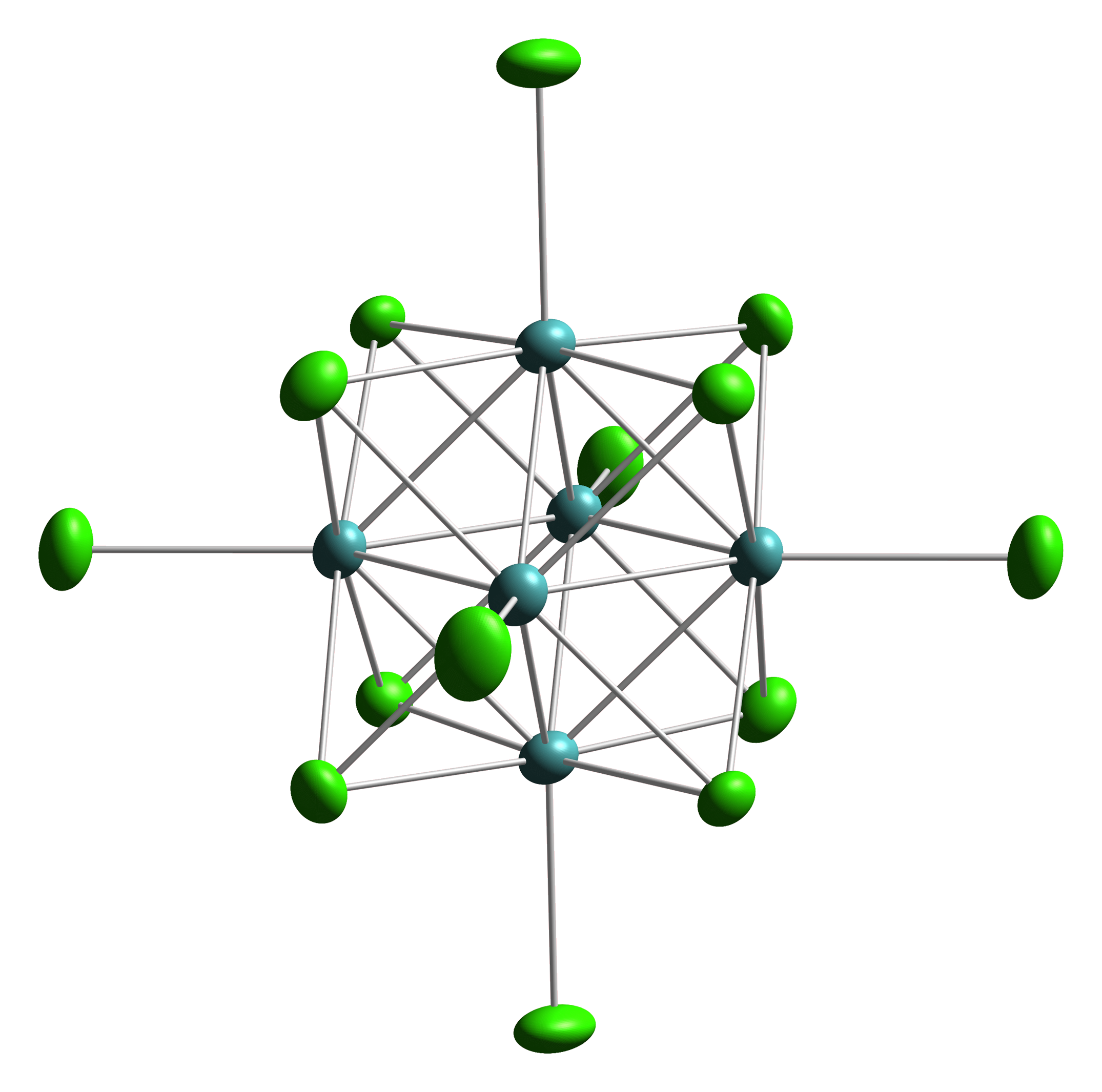
Tin(II) hydroxide, Sn(OH)2, also known as ''stannous hydroxide'', is an inorganic compound tin(II). The only related material for which definitive information is available is the oxy hydroxide Sn6O4(OH)4, but other related materials are claimed. They are all white solids that are insoluble in water. Preparation and structure Crystals of Sn6O4(OH)4 has been characterized by X-ray diffraction. This cluster is obtained from solution of basic solutions of tin(II). The compound consists of an octahedron of Sn centers, each face of which is capped by an oxide or a hydroxide. The structure is reminiscent of the Mo6S8 subunit of the Chevrel phase Octahedral clusters are inorganic or organometallic cluster compounds composed of six metals in an octahedral array.Eric J. Welch and Jeffrey R. Long ''Atomlike Building Units of Adjustable Character: Solid-State and Solution Routes to Manipulating ...s.. The structure of pure Sn(OH)2 is not known. Sn(OH)2 has been claimed to arise from the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chevrel Phase
Octahedral clusters are inorganic or organometallic cluster compounds composed of six metals in an octahedral array.Eric J. Welch and Jeffrey R. Long ''Atomlike Building Units of Adjustable Character: Solid-State and Solution Routes to Manipulating Hexanuclear Transition Metal Chalcohalide Clusters'' in Progress in Inorganic Chemistry, Volume 54 Kenneth D. Karlin 2005''Link/ref> Many types of compounds are known, but all are synthetic. Octahedral chalcogenide and halide clusters These compounds are bound together by metal-metal bonding as well as two kinds of ligands. Ligands that span the faces or edges of the M6 core are labeled Li, for ''inner'' (innen in the original German description), and those ligands attached only to one metal are labeled outer, or La for ''ausser''. Typically, the outer ligands can be exchanged whereas the bridging ligands are more inert toward substitution. Face-capped halide clusters The premier example is of the class is Mo6Cl142−. This dianion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stannic Acid
Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid. Structure Tin(IV) oxide crystallises with the rutile structure. As such the tin atoms are six coordinate and the oxygen atoms three coordinate. SnO2 is usually regarded as an oxygen-deficient n-type semiconductor. Hydrous forms of SnO2 have been described as stannic acid. Such materials appear to be hydrated particles of SnO2 where the composition reflects the particle size. Preparation Tin(IV) oxide occurs naturally. Synthetic tin(IV) oxide is produced by burning tin metal in air. Annual production is in the range of 10 kilotons. SnO2 is reduced industrially to the metal with carbon in a reverberatory furnace at 1200–1300 °C. Amphoterism Although SnO2 is insoluble ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxides
Hydroxide is a polyatomic ion, diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually Self-ionization of water, minor constituent of water. It functions as a base (chemistry), base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salt (chemistry), salts, some of which dissociation (chemistry), dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemicals, commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalent bond, covalently bound functional group, group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry. Many inorganic substances which bear the word ''hydroxide'' in their names ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tin(II) Compounds
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal. Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, the so-called "tin cry" can be heard as a result of twinning in tin crystals; this trait is shared by indium, cadmium, zinc, and mercury in the solid state. Pure tin after solidifying presents a mirror-like appearance similar to most metals. In most tin alloys (such as pewter) the metal solidifies with a dull gray color. Tin is a post-transition metal in group 14 of the periodic table of elements. It is obtained chiefly from the mineral cassiterite, which contains stannic oxide, . Tin shows a chemical similarity to both of its neighbors in group 14, germanium and lead, and has two main oxidation states, +2 and the slightly more stable +4. Tin is the 49th most abundant element on Earth and has, with 10 stable isotopes, the larges ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |