|
Tetramethylurea
Tetramethylurea is the organic compound with the formula (Me2N)2CO. It is a substituted urea. This colorless liquid is used as an aprotic-polar solvent, especially for aromatic compounds and is used e. g. for Grignard reagents. Production The synthesis and properties of tetramethylurea were comprehensively described. The reaction of dimethylamine with phosgene in the presence of e. g. 50 % sodium hydroxide solution and subsequent extraction with 1,2-dichloroethane yields tetramethylurea in 95% yield. The reactions with dimethylcarbamoyl chloride or phosgene are highly exothermic and the removal of the resulting dimethylamine hydrochloride requires some effort. The reaction of diphenylcarbonate with dimethylamine in an autoclave is also effective. Tetramethylurea is formed in the reaction of dimethylcarbamoyl chloride with anhydrous sodium carbonate in a yield of 96.5%. Dimethylcarbamoyl chloride also reacts with excess dimethylamine forming tetramethylurea. Even thoug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylcarbamoyl Chloride
Dimethylcarbamoyl chloride (DMCC) is a reagent for transferring a dimethylcarbamoyl group to alcoholic or phenolic hydroxyl groups forming dimethyl carbamates, usually having pharmacological or pesticidal activities. Because of its high toxicity and its carcinogenic properties shown in animal experiments and presumably also in humans, dimethylcarbamoyl chloride can only be used under stringent safety precautions. Production and occurrence The production of dimethylcarbamoyl chloride from phosgene and dimethylamine was reported as early as 1879 (reported as "Dimethylharnstoffchlorid" – dimethylurea chloride). : DMCC can be produced in high yields (90%) at 275 °C by reacting phosgene with gaseous dimethylamine in a flow reactor. To suppress the formation of ureas, excess phosgene is used (in a 3:1 ratio). The reaction can also be carried out at the laboratory scale with diphosgene or triphosgene and an aqueous dimethylamine solution in the two-phase system of benzene–xy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
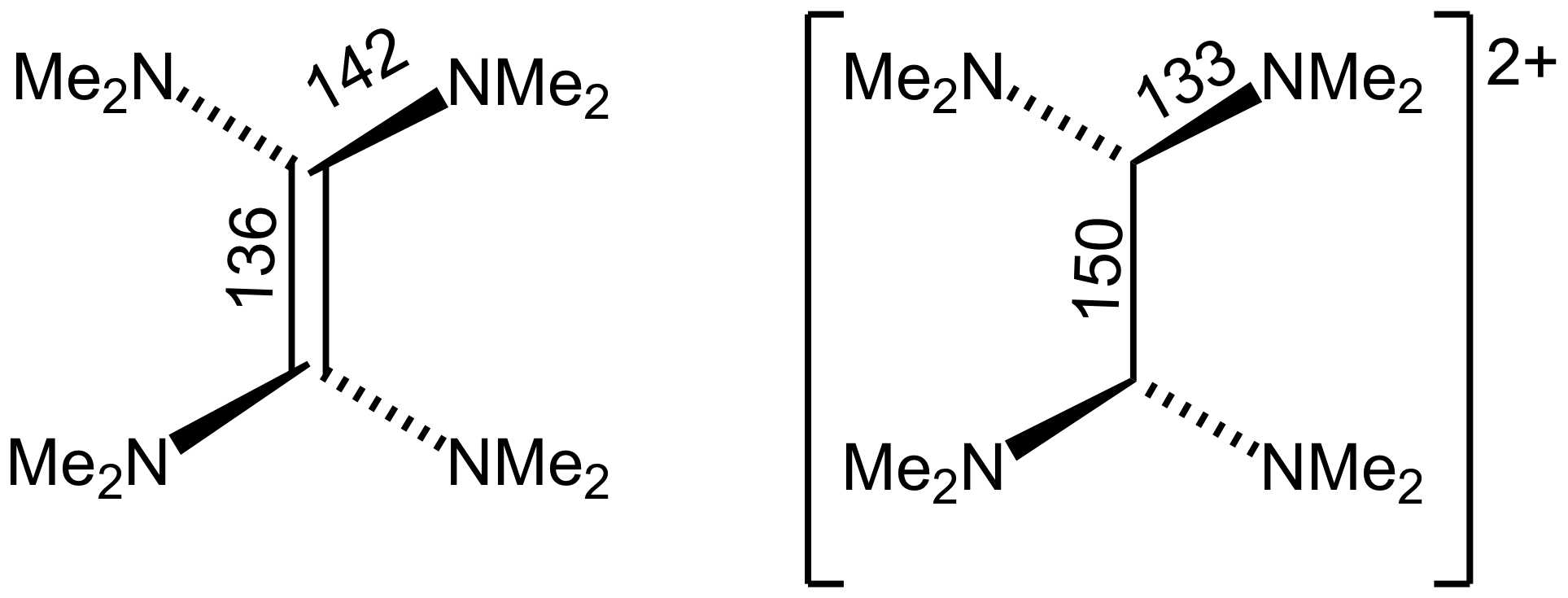
Tetrakis(dimethylamino)ethylene
Tetrakis(dimethylamino)ethylene (TDAE) is an organic compound with the formula (NMe2)2sub>2 (where Me = CH3). A colorless liquid, this compound is classified as an enamine. Primary and secondary enamines tend to isomerize, but tertiary enamines are kinetically stable. The unusual feature of TDAE is that it is a tetra-enamine. The pi-donating tendency of the amine groups strongly modifies the properties of the molecule, which does exhibit properties of a typical alkene. Reactions TDAE reacts with oxygen in a chemiluminescent reaction to give tetramethylurea TDAE is an electron donor with E = 1.06 V vs Fc+/0. It forms a charge transfer salt with buckminsterfullerene: :C2(N(CH3)2)4 + C60 → 2(N(CH3)2)4+C60−] Oxidation affords a dication. Structure Crystallographic analysis show that TDAE is a highly distorted alkene, the dihedral angle for the two N2C termini is 28″. The C=C distance is alkene-like, 135 pm. The nearly isostructural tetraisopropylethylene also has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris(dimethylamino)methane
Tris(dimethylamino)methane (TDAM) is the simplest representative of the tris(dialkylamino)methanes of the general formula (R2N)3CH in which three of the four of methane's hydrogen atoms are replaced by dimethylamino groups (−N(CH3)2). Tris(dimethylamino)methane can be regarded as both an amine and an orthoamide. Tris(dimethylamino)methane is a strong base and can be used as a formylation agent, as aminomethylenation reagent and as a source for the basic bis(dimethylamino)carbene of the formula (R2N)2C:. Preparation Tris(dimethylamino)methane is formed in the reaction of N,N,N′,N′-Tetramethylformamidinium chloride (TMF-Cl) or bis(dimethylamino)acetonitrile with lithium dimethylamide or sodium dimethylamide with yields between 55 and 84%. :: From dimethylamine and trimethoxyborane sodium dimethylamide is formed ''in situ'' in the presence of sodium hydride which reacts with ''N'',''N'',''N'',''N''-tetramethylformamidinium chloride in 84% yield to tris(dimethylamino) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrakis(dimethylamino)ethylene
Tetrakis(dimethylamino)ethylene (TDAE) is an organic compound with the formula (NMe2)2sub>2 (where Me = CH3). A colorless liquid, this compound is classified as an enamine. Primary and secondary enamines tend to isomerize, but tertiary enamines are kinetically stable. The unusual feature of TDAE is that it is a tetra-enamine. The pi-donating tendency of the amine groups strongly modifies the properties of the molecule, which does exhibit properties of a typical alkene. Reactions TDAE reacts with oxygen in a chemiluminescent reaction to give tetramethylurea TDAE is an electron donor with E = 1.06 V vs Fc+/0. It forms a charge transfer salt with buckminsterfullerene: :C2(N(CH3)2)4 + C60 → 2(N(CH3)2)4+C60−] Oxidation affords a dication. Structure Crystallographic analysis show that TDAE is a highly distorted alkene, the dihedral angle for the two N2C termini is 28″. The C=C distance is alkene-like, 135 pm. The nearly isostructural tetraisopropylethylene also has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions in water. Historically, it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process. Hydrates Sodium carbonate is obtained as three hydrates and as the anhydrous salt: * sodium carbonate decahydrate (natron), Na2CO3·10H2O, which readily efflorescence, effloresces to form the monohydrate. * sodium carbonate heptahydrate (not known in mineral form), Na2CO3·7H2O. * sodium carbonate monohydrate (thermonatrite), Na2CO3·H2O. Also known as crystal carbonate. * anhydrous sodium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats. The global ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation Von TDAE
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogenation, C=C (and other) bonds ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloaddition Reaction
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". The resulting reaction is a cyclization reaction. Many but not all cycloadditions are concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile. Cycloadditions can be described using two systems of notation. An older but still common notation is based on the size of linear arrangements of atoms in the reactants. It uses parentheses: where the variables are the numbers of linear atoms in each reactant. The product is a cycle of size . In this system, the standard Diels-Alder reaction is a (4 + 2)-cycloaddition, the 1,3-dipolar cycloaddition is a (3 + 2)-cycloadditio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TDAE Aus Chlortrifluorethen
''Total Drama Island'' (sometimes shortened to ''TDI'') is the first season of ''Total Drama'', a Canadian animated comedy television series created by Tom McGillis and Jennifer Pertsch. The series premiered in Canada on Teletoon on July 8, 2007, and ran for 26 episodes, each 22 minutes in duration with a special 44-minute season finale. Plot ''Total Drama Island'' is set in the fictional titular reality show, which follows the competition of 22 unsuspecting and unwitting teenagers at Camp Wawanakwa, the most rundown, insect-infested, disgusting island in an unspecified area in Muskoka, Ontario. The campers participate in competitions and challenges that get more insane and dangerous each week to avoid being voted off the island by their fellow campers and teammates. At the end of the series, the winning contestant will receive Canadian dollar, C$100,000 (United States dollar, US$73,129.00). The competition is hosted by Chris McLean, assisted by the camp's chef, Chef Hatchet, wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorotrifluoroethene
Chlorotrifluoroethylene (CTFE) is a chlorofluorocarbon with chemical formula CFCl=CF2. It is commonly used as a refrigerant in cryogenic applications. CTFE has a carbon-carbon double bond and so can be polymerized to form polychlorotrifluoroethylene or copolymerized to produce the plastic ECTFE. PCTFE has the trade name Neoflon PCTFE from Daikin Industries in Japan, and it used to be produced under the trade name Kel-F from 3M Corporation in Minnesota. Production and reactions Chlorotrifluoroethylene is produced commercially by the dechlorination of 1,1,2-trichloro-1,2,2-trifluoroethane with zinc: :CFCl2-CF2Cl + Zn → CClF=CF2 + ZnCl2 In 2012, an estimated 1–10 million pounds were produced commercially in the United States. The thermal dimerization of chlorotrifluoroethylene gives 1,2-dichloro-1,2,3,3,4,4-hexafluorocyclobutane. Dichlorination of the latter gives hexafluorocyclobutene Hexafluorocyclobutene is the organofluorine compound with the formula (CF2)2(CF)2. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |