|
Tetracobalt Dodecacarbonyl
Tetracobalt dodecacarbonyl is the chemical compound with the formula Co4(CO)12. It is a black crystalline compound that is insoluble in water and easily oxidized by air. It is an example of a metal carbonyl cluster In chemistry, a metal carbonyl cluster is a compound that contains two or more metals linked in part by metal-metal bonds and containing carbon monoxide (CO) as the exclusive or predominant ligand. The area is a subfield of metal carbonyl chemistr .... Synthesis and structure This compound is synthesized by decarbonylation of Co2(CO)8. :2 Co2(CO)8 → Co4(CO)12 + 4 CO The molecule consists of a tetrahedral Co4 core, but the molecular symmetry is C3v. Three carbonyl ligands are bridging ligands and nine are terminal. The average Co-Co distance is 2.499 Å, the average C-O bond length is 1.133 Å, and the average Co-C-O angle is 177.5°. Rh4(CO)12 adopts the same C3v structure but Ir4(CO)12 has perfect Td symmetry with no bridging CO ligands groups. The Rh4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Carbonyl Cluster
In chemistry, a metal carbonyl cluster is a compound that contains two or more metals linked in part by metal-metal bonds and containing carbon monoxide (CO) as the exclusive or predominant ligand. The area is a subfield of metal carbonyl chemistry, and many metal carbonyl clusters are in fact prepared from simple metal carbonyls. Simple examples include Fe2(CO)9, Fe3(CO)12, Mn2(CO)10. High nuclearity clusters include h13(CO)24H3sup>2− and the stacked Pt3 triangules t3n(CO)6nsup>2− (n = 2–6). History The first metal carbonyl clusters, Fe3(CO)12, Ir4(CO)12, and Rh6(CO)16, were reported starting in the 1930s, often by Walter Hieber. The structures were subsequently established by X-ray crystallography.. Paolo Chini Paolo Chini (1928–1980) was an Italian chemist, known as the "King of the Clusters". He was a pioneer in metal carbonyl cluster syntheses. He developed and improved quantitative methods for the synthesis of large carbonyl clusters, such as t ... ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicobalt Octacarbonyl
Dicobalt octacarbonyl is an organocobalt compound with composition . This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry. It is the parent member of a family of hydroformylation catalysts. Each molecule consists of two cobalt atoms bound to eight carbon monoxide ligands, although multiple structural isomers are known. Some of the carbonyl ligands are labile. Synthesis, structure, properties Dicobalt octacarbonyl an orange-colored, pyrophoric solid. It is synthesised by the high pressure carbonylation of cobalt(II) salts: : The preparation is often carried out in the presence of cyanide, converting the cobalt(II) salt into a hexacyanocobaltate(II) complex that reacts with carbon monoxide to yield . Acidification produces cobalt tetracarbonyl hydride, , which degrades near room temperature to dicobalt octacarbonyl and hydrogen. It can also be prepared by heating cobalt metal to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Ligand
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes. Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of oxygen. Nomenclature and terminology The nomenclature of the metal carbonyls depends on the charge of the complex, the number and type of central atoms, and the number and type of ligands and their binding modes. They occur as neutral complexes, as positively-charged metal carbonyl cations or as negatively charged metal carbonylates. The carbon monoxide liga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
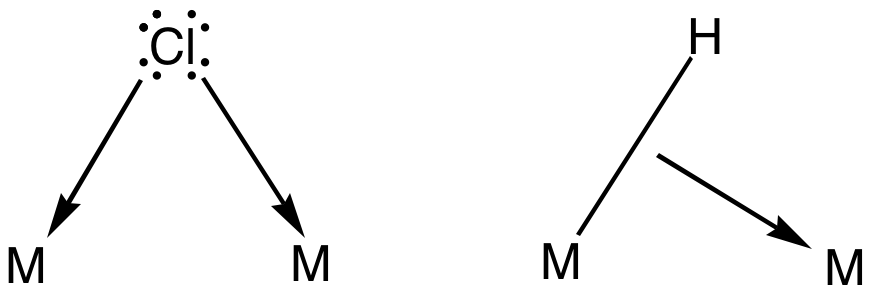
Bridging Ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals. In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek letter mu, μ, with a subscript number denoting the number of metals bound to the bridging ligand. μ2 is often denoted simply as μ. When describing coordination complexes care should be taken not to confuse μ with η ('eta'), which relates to hapticity. Ligands that are not bridging are called terminal ligands. List of bridging ligands Virtually all ligands are known to bridge, with the exception of amines and ammonia. Common bridging ligands include most of the common anions. Many simple organic ligands form str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganica Chimica Acta
''Inorganica Chimica Acta'' is a peer-reviewed scientific journal published since 1967 that covers original research and reviews of fundamental and applied aspects of inorganic chemistry Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disci .... See also * List of scientific journals in chemistry External links * Elsevier academic journals Inorganic chemistry journals Publications established in 1967 English-language journals Journals published between 13 and 25 times per year {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Organometallic Chemistry
The ''Journal of Organometallic Chemistry'' is a peer-reviewed scientific journal published by Elsevier, covering research on organometallic chemistry. According to the ''Journal Citation Reports'', the journal has a 2021 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as i ... of 2.345. References External links * Organic chemistry journals Elsevier academic journals Publications established in 1964 English-language journals Monthly journals {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Chemical Physics
''The Journal of Chemical Physics'' is a scientific journal published by the American Institute of Physics that carries research papers on chemical physics."About the Journal" from the ''Journal of Chemical Physics'' website. Two volumes, each of 24 issues, are published annually. It was established in 1933 when '''' editors refused to publish theoretical works. The editors have been: *2019-present: Tim Lian *2008–2018: *2007–2008: [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrarhodium Dodecacarbonyl
Tetrarhodium dodecacarbonyl is the chemical compound with the formula Rh4(CO)12. This dark-red crystalline solid is the smallest binary rhodium carbonyl that can be handled as a solid under ambient conditions. It is used as a catalyst in organic synthesis. Structure, synthesis, reactions According to X-ray crystallography, features a tetrahedral array of four Rh atoms with nine terminal CO ligands and three bridging CO ligands. The structure can be expressed as Rh4(CO)9(µ-CO)3. is prepared by treatment of an aqueous solution of rhodium trichloride with activated copper metal under an atmosphere of CO. :4 RhCl3(H2O)3 + 8 Cu + 22 CO → + 2 CO2 + 8 Cu(CO)Cl + 4 HCl + 10 H2O Alternatively, the compound can be prepared by treatment of a methanolic solution of RhCl3(H2O)3 with CO to afford H hCl2(CO)2 followed by carbonylation in the presence ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrairidium Dodecacarbonyl
Tetrairidium dodecacarbonyl is the chemical compound with the formula Ir4(CO)12. This tetrahedral cluster is the most common and most stable "binary" carbonyl of iridium. This air-stable species is only poorly soluble in organic solvents. It has been used to prepare bimetallic clusters and catalysts, e.g. for the water gas shift reaction, and reforming, but these studies are of purely academic interest. Structure Each Ir center is octahedral, being bonded to 3 other iridium atoms and three terminal CO ligands. Ir4(CO)12 has Td symmetry with an average Ir-Ir distances of 2.693 Å. The related clusters Rh4(CO)12 and Co4(CO)12 have C3v symmetry because of the presence of three bridging CO ligands in each. Preparation It is prepared in two steps by reductive carbonylation of hydrated iridium trichloride Iridium(III) chloride is the inorganic compound with the formula IrCl3. The anhydrous compound is relatively rare, but the related hydrate is useful for preparing other iri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Chemistry (journal)
''Inorganic Chemistry'' is a biweekly peer-reviewed scientific journal published by the American Chemical Society since 1962. It covers research in all areas of inorganic chemistry. The current editor-in-chief is William B. Tolman (Washington University). Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2021 impact factor of 5.436. See also * ''Organometallics ''Organometallics'' is a biweekly journal published by the American Chemical Society. Its area of focus is organometallic and organometalloid chemistry. This peer-reviewed journal has an impact factor of 3.837 as reported by the 2021 Journal Cit ...'' References External links * American Chemical Society academic journals Biweekly journals Publications established in 1962 English-language journals Inorganic chemistry journals {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobalt Complexes
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal. Cobalt-based blue pigments (cobalt blue) have been used since ancient times for jewelry and paints, and to impart a distinctive blue tint to glass, but the color was for a long time thought to be due to the known metal bismuth. Miners had long used the name ''kobold ore'' (German for ''goblin ore'') for some of the blue-pigment-producing minerals; they were so named because they were poor in known metals, and gave poisonous arsenic-containing fumes when smelted. In 1735, such ores were found to be reducible to a new metal (the first discovered since ancient times), and this was ultimately named for the ''kobold''. Today, some cobalt is produced specifically from one of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

8NoCo-Co.png)