|
Tepotinib
Tepotinib, sold under the brand name Tepmetko, is an anti-cancer medication used for the treatment of adults with non-small cell lung cancer (NSCLC). The most common side effects include edema (build-up of fluid), nausea (feeling sick), low albumin level in the blood, diarrhea, and increase in creatinine level in the blood (a sign of kidney problems). Tepotinib first received marketing approval in Japan, in March 2020, as a "line-agnostic" drug, meaning it is approved both for treatment-naive patients and for those in whom previous attempts at treatment have failed. US approval followed in February 2021. It is the second therapy approved by the US Food and Drug Administration (FDA) to treat non-small cell lung cancer with these particular mutations, after capmatinib. Medical uses Tepotinib is indicated for the treatment of adults with metastatic non-small cell lung cancer (NSCLC) whose tumors have a mutation that leads to mesenchymal-epithelial transition (MET) exon 14 skip ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dyspnea
Shortness of breath (SOB), also medically known as dyspnea (in AmE) or dyspnoea (in BrE), is an uncomfortable feeling of not being able to breathing, breathe well enough. The American Thoracic Society defines it as "a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity", and recommends evaluating dyspnea by assessing the intensity of its distinct sensations, the degree of distress and discomfort involved, and its burden or impact on the patient's activities of daily living. Distinct sensations include effort/work to breathe, chest tightness or pain, and "air hunger" (the feeling of not enough oxygen). The tripod position is often assumed to be a sign. Dyspnea is a normal symptom of heavy physical exertion but becomes disease, pathological if it occurs in unexpected situations, when resting or during light exertion. In 85% of cases it is due to asthma, pneumonia, cardiac ischemia, interstitial lung disease, congesti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breakthrough Therapy
Breakthrough therapy is a United States Food and Drug Administration designation that expedites drug development that was created by Congress under Section 902 of the 9 July 2012 Food and Drug Administration Safety and Innovation Act. The FDA's "breakthrough therapy" designation is not intended to imply that a drug is actually a "breakthrough" or that there is high-quality evidence of treatment efficacy for a particular condition; rather, it allows the FDA to grant priority review to drug candidates if preliminary clinical trials indicate that the therapy may offer substantial treatment advantages over existing options for patients with serious or life-threatening diseases. The FDA has other mechanisms for expediting the review and approval process for promising drugs, including fast track designation, accelerated approval, and priority review. Requirements A breakthrough therapy designation can be assigned to a drug if "it is a drug which is intended alone or in combination with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antineoplastic Drugs
Chemotherapy (often abbreviated to chemo and sometimes CTX or CTx) is a type of cancer treatment that uses one or more anti-cancer drugs (chemotherapeutic agents or alkylating agents) as part of a standardized chemotherapy regimen. Chemotherapy may be given with a curative intent (which almost always involves combinations of drugs) or it may aim to prolong life or to reduce symptoms ( palliative chemotherapy). Chemotherapy is one of the major categories of the medical discipline specifically devoted to pharmacotherapy for cancer, which is called ''medical oncology''. The term ''chemotherapy'' has come to connote non-specific usage of intracellular poisons to inhibit mitosis (cell division) or induce DNA damage, which is why inhibition of DNA repair can augment chemotherapy. The connotation of the word chemotherapy excludes more selective agents that block extracellular signals ( signal transduction). The development of therapies with specific molecular or genetic targets, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. The conditions are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy in many countries and has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug research and development. In the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to encourage development of drugs which would otherwise lack sufficient profit motive to attract corporate research budgets and personnel. Definition According to the US Food and Drug Administration (FDA), an orphan drug is defined as one "intended for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committee For Medicinal Products For Human Use
The Committee for Medicinal Products for Human Use (CHMP), formerly known as Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding medicinal products for human use. See also * Committee for Medicinal Products for Veterinary Use The Committee for Medicinal Products for Veterinary Use (CVMP) is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding veterinary medicines. Text was copied from this source which is © ... References External links Committee for Medicinal Products for Human Use (CHMP) Health and the European Union {{eu-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Embryotoxicity
Teratology is the study of abnormalities of physiological development in organisms during their life span. It is a sub-discipline in medical genetics which focuses on the classification of congenital abnormalities in dysmorphology. The related term developmental toxicity includes all manifestations of abnormal development that are caused by environmental insult. These may include growth retardation, delayed mental development or other congenital disorders without any structural malformations. Teratogens are substances that may cause birth defects via a toxic effect on an embryo or fetus. Known teratogens include: retinol, thalidomide, mercury, alcohol, lead, polychlorinated biphenyls (PCBs), and 2,3,7,8-tetrachlorodibenzodioxin. Etymology The term was borrowed in 1842 from the French , where it was formed in 1830 from the Greek (word stem ), meaning "sign sent by the gods, portent, marvel, monster", and (''-ology''), used to designate a discourse, treaty, science, theory, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hepatotoxicity
Hepatotoxicity (from ''hepatic toxicity'') implies chemical-driven liver damage. Drug-induced liver injury is a cause of acute and chronic liver disease caused specifically by medications and the most common reason for a drug to be withdrawn from the market after approval. The liver plays a central role in transforming and clearing chemicals and is susceptible to the toxicity from these agents. Certain medicinal agents, when taken in overdoses (e.g. paracetamol) and sometimes even when introduced within therapeutic ranges (e.g. halothane), may injure the organ. Other chemical agents, such as those used in laboratories and industries, natural chemicals (e.g., microcystins), and herbal remedies (two prominent examples being kava, mechanism unknown, and comfrey, through its pyrrolizidine alkaloid content) can also induce hepatotoxicity. Chemicals that cause liver injury are called hepatotoxins. More than 900 drugs have been implicated in causing liver injury (see LiverTox, exter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
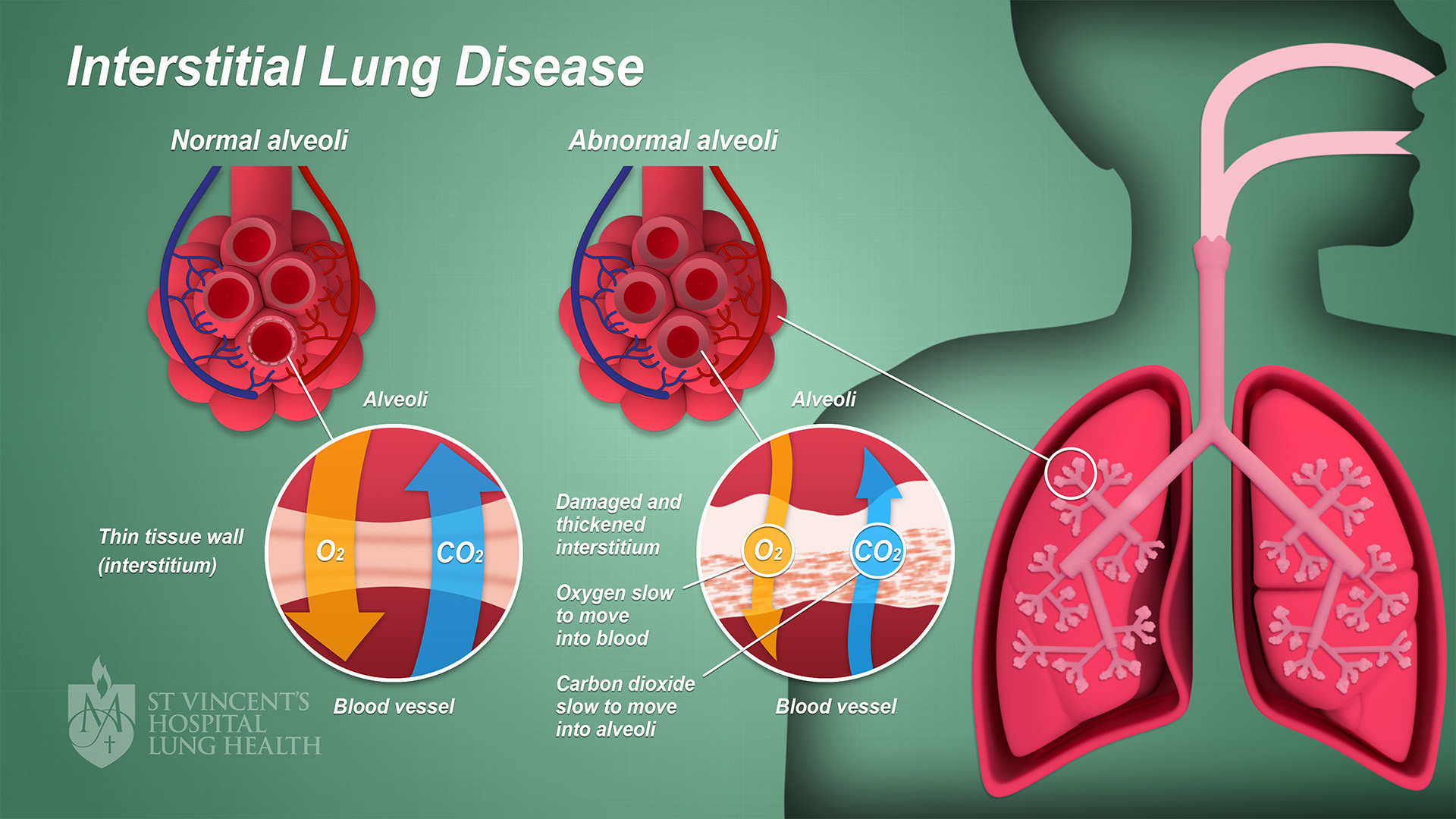
Interstitial Lung Disease
Interstitial lung disease (ILD), or diffuse parenchymal lung disease (DPLD), is a group of respiratory diseases affecting the interstitium (the tissue and space around the alveoli (air sacs)) of the lungs. It concerns alveolar epithelium, pulmonary capillary endothelium, basement membrane, and perivascular and perilymphatic tissues. It may occur when an injury to the lungs triggers an abnormal healing response. Ordinarily, the body generates just the right amount of tissue to repair damage, but in interstitial lung disease, the repair process is disrupted, and the tissue around the air sacs (alveoli) becomes scarred and thickened. This makes it more difficult for oxygen to pass into the bloodstream. The disease presents itself with the following symptoms: shortness of breath, nonproductive coughing, fatigue, and weight loss, which tend to develop slowly, over several months. The average rate of survival for someone with this disease is between three and five years. The term ILD i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exon
An exon is any part of a gene that will form a part of the final mature RNA produced by that gene after introns have been removed by RNA splicing. The term ''exon'' refers to both the DNA sequence within a gene and to the corresponding sequence in RNA transcripts. In RNA splicing, introns are removed and exons are covalently joined to one another as part of generating the mature RNA. Just as the entire set of genes for a species constitutes the genome, the entire set of exons constitutes the exome. History The term ''exon'' derives from the expressed region and was coined by American biochemist Walter Gilbert in 1978: "The notion of the cistron… must be replaced by that of a transcription unit containing regions which will be lost from the mature messengerwhich I suggest we call introns (for intragenic regions)alternating with regions which will be expressedexons." This definition was originally made for protein-coding transcripts that are spliced before being translated. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anti-cancer Medication
Chemotherapy (often abbreviated to chemo and sometimes CTX or CTx) is a type of cancer treatment that uses one or more anti-cancer drugs (chemotherapeutic agents or alkylating agents) as part of a standardized chemotherapy regimen. Chemotherapy may be given with a curative intent (which almost always involves combinations of drugs) or it may aim to prolong life or to reduce symptoms (palliative chemotherapy). Chemotherapy is one of the major categories of the medical discipline specifically devoted to pharmacotherapy for cancer, which is called ''medical oncology''. The term ''chemotherapy'' has come to connote non-specific usage of intracellular poisons to inhibit mitosis (cell division) or induce DNA damage, which is why inhibition of DNA repair can augment chemotherapy. The connotation of the word chemotherapy excludes more selective agents that block extracellular signals (signal transduction). The development of therapies with specific molecular or genetic targets, whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |