|
Tantalum Pentoxide
Tantalum pentoxide, also known as tantalum(V) oxide, is the inorganic compound with the formula . It is a white solid that is insoluble in all solvents but is attacked by strong bases and hydrofluoric acid. is an inert material with a high refractive index and low absorption (i.e. colourless), which makes it useful for coatings. It is also extensively used in the production of capacitors, due to its high dielectric constant. Preparation Occurrence Tantalum occurs in the minerals tantalite and columbite (columbium being an archaic name for niobium), which occur in pegmatites, an igneous rock formation. Mixtures of columbite and tantalite are called coltan. Tantalite was discovered by Anders Gustaf Ekeberg at Ytterby, Sweden, and Kimoto, Finland. The minerals microlite and pyrochlore contain approximately 70% and 10% Ta, respectively. Refining Tantalum ores often contain significant amounts of niobium, which is itself a valuable metal. As such, both metals are extracted so that th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as ''tantalium'', it is named after Tantalus, a villain in Greek mythology. Tantalum is a very hard, ductile, lustrous, blue-gray transition metal that is highly corrosion-resistant. It is part of the refractory metals group, which are widely used as components of strong high-melting-point alloys. It is a group 5 element, along with vanadium and niobium, and it always occurs in geologic sources together with the chemically similar niobium, mainly in the mineral groups tantalite, columbite and coltan. The chemical inertness and very high melting point of tantalum make it valuable for laboratory and industrial equipment such as reaction vessels and vacuum furnaces. It is used in tantalum capacitors for electronic equipment such as computers. Tantalum is considered a technology-critical element by the European Commission. History Tantalum was discovered in Sweden in 1802 by Anders ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microlite
Microlite was once known as a pale-yellow, reddish-brown, or black isometric mineral composed of sodium calcium tantalum oxide with a small amount of fluorine. Its chemical formula is. Today it is a name of a group of oxide minerals of a similar stoichiometry having tantalum prevailing over titanium and niobium. The microlite group belongs to a large pyrochlore supergroup that occurs in pegmatites and constitutes an ore of tantalum. It has a Mohs hardness of 5.5 and a variable specific gravity of 4.2 to 6.4. It occurs as disseminated microscopic subtranslucent to opaque octahedral crystals with a refractive index of 2.0 to 2.2. Microlite is also called djalmaite, but both names are now obsolete. "Microlite" occurs as a primary mineral in lithium-bearing granite pegmatites, and in miarolitic cavities in granites. Association minerals include: albite, lepidolite, topaz, beryl, tourmaline, spessartine, tantalite and fluorite. "Microlite" was first described in 1835 for an occurr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Solvents
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical synth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
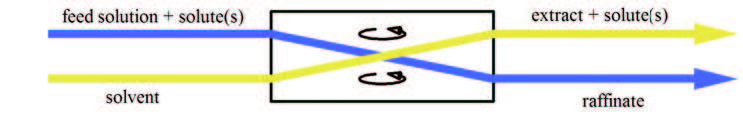
Liquid–liquid Extraction
Liquid–liquid extraction (LLE), also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more species from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration (lower free energy). The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. LLE is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as mixer settlers. This type of process is commonly performed after ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aqueous
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be represented as . The word ''aqueous'' (which comes from ''aqua'') means pertaining to, related to, similar to, or dissolved in, water. As water is an excellent solvent and is also naturally abundant, it is a ubiquitous solvent in chemistry. Since water is frequently used as the solvent in experiments, the word solution refers to an aqueous solution, unless the solvent is specified. A ''non-aqueous solution'' is a solution in which the solvent is a liquid, but is not water. (See also Solvent and Inorganic nonaqueous solvent.) Characteristics Substances that are ''hydrophobic'' ('water-fearing') do not dissolve well in water, whereas those that are ''hydrophilic'' ('water-friendly') do. An example of a hydrophilic substance is sodium chlo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese(II) Fluoride
Manganese(II) fluoride is the chemical compound composed of manganese and fluoride with the formula MnF2. It is a light pink solid, the light pink color being characteristic for manganese(II) compounds. It is made by treating manganese and diverse compounds of manganese(II) in hydrofluoric acid. Like some other metal difluorides, MnF2 crystallizes in the rutile structure, which features octahedral Mn centers. Uses MnF2 is used in the manufacture of special kinds of glass and laser A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word "laser" is an acronym for "light amplification by stimulated emission of radiation". The ...s. It is a canonical example of uniaxial antiferromagnet (with Neel temperature of 68 K) which has been experimentally studied since early on. References Manganese(II) compounds Fluorides Metal halides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(II) Fluoride
Iron(II) fluoride or ferrous fluoride is an inorganic compound with the molecular formula FeF2. It forms a tetrahydrate FeF2·4H2O that is often referred to by the same names. The anhydrous and hydrated forms are white crystalline solids.Dale L. Perry (1995),Handbook of Inorganic Compounds, page 167. CRC Press. Structure and bonding Anhydrous FeF2 adopts the TiO2 rutile structure. As such, the iron cations are octahedral and fluoride anions are trigonal planar. The tetrahydrate can exist in two structures, or polymorphs. One form is rhombohedral and the other is hexagonal, the former having a disorder. Like most fluoride compounds, the anhydrous and hydrated forms of iron(II) fluoride feature high spin metal center. Low temperature neutron diffraction studies show that the FeF2 is antiferromagnetic. Heat capacity measurements reveal an event at 78.3 K corresponding to ordering of antiferromagnetic state. Selected physical properties FeF2 sublimes between 958 and 1178 K. Usi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Heptafluorotantalate
Potassium heptafluorotantalate is an inorganic compound with the formula K2 aF7 It is the potassium salt of the heptafluorotantalate anion aF7sup>2−. This white, water-soluble solid is an intermediate in the purification of tantalum from its ores and is the precursor to the metal. Preparation Industrial Potassium heptafluorotantalate is an intermediate in the industrial production of metallic tantalum. Its production involves leaching tantalum ores, such as columbite and tantalite, with hydrofluoric acid and sulfuric acid to produce the water-soluble hydrogen pentafluorotantalate. : Ta2O5 + 14 HF → 2 H2 aF7 + 5 H2O This solution is subjected to a number of liquid-liquid extraction steps to remove metallic impurities (most importantly niobium) before being treated with potassium fluoride to produce K2 aF7 : H2 aF7+ 2 KF → K2 aF7+ 2 HF Lab-scale Hydrofluoric acid is both corrosive and toxic, making it unappealing to work with; as such a number of alternative ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, e.g. polytetrafluoroethylene (PTFE). HF is widely used in the petrochemical industry as a component of superacids. Hydrogen fluoride boils at near room temperature, much higher than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid had been known in the glass industry before then. French chemist Edmond Frémy (1814–1894) is credited with discov ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluoric Acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly corrosive. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers. Uses Production of organofluorine compounds The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Teflon, fluoropolymers, fluorocarbons, and refrigerants such as freon. Many pharmaceuticals contain fluorine. Production of inorganic fluorides Most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost are Na3AlF6, cryolite, and AlF3, aluminium trifluoride. A molten mixture of these solids serves as a high-temperature solvent for the prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leaching (metallurgy)
Leaching is a process widely used in extractive metallurgy where ore is treated with chemicals to convert the valuable metals within into soluble salts while the impurity remains insoluble. These can then be washed out and processed to give the pure metal; the materials left over are commonly known as tailings. Compared to pyrometallurgy, leaching is easier to perform, requires less energy and is potentially less harmful as no gaseous pollution occurs. Drawbacks of leaching include its lower efficiency and the often significant quantities of waste effluent and tailings produced, which are usually either highly acidic or alkali as well as toxic (e.g. bauxite tailings). There are four types of leaching: # Cyanide leaching (e.g. gold ore) # Ammonia leaching (e.g. crushed ore) # Alkali leaching (e.g. bauxite ore) # Acid leaching (e.g. sulfide ore) Chemistry Leaching is done in long pressure vessels which are cylindrical (horizontal or vertical) or of horizontal tube form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



_0468.jpg)