|
Tyrosinaemia
Tyrosinemia or tyrosinaemia is an error of metabolism, usually inborn, in which the body cannot effectively break down the amino acid tyrosine. Symptoms of untreated tyrosinemia include liver and kidney disturbances. Without treatment, tyrosinemia leads to liver failure. Today, tyrosinemia is increasingly detected on newborn screening tests before any symptoms appear. With early and lifelong management involving a low-protein diet, special protein formula, and sometimes medication, people with tyrosinemia develop normally, are healthy, and live normal lives. Cause All tyrosinemias result from dysfunction of various genes in the phenylalanine and tyrosine catabolic pathway, and are inherited in an autosomal-recessive pattern. Type I tyrosinemia results from a mutation in the ''FAH'' gene, which encodes the enzyme fumarylacetoacetase. As a result of ''FAH'' deficiency, the substrate fumarylacetoacetate can accumulate in proximal renal tubular cells and hepatocytes, resulting in d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Type I Tyrosinemia
Tyrosinemia type I is a genetic disorder that disrupts the metabolism of the amino acid tyrosine, resulting in damage primarily to the liver along with the kidneys and peripheral nerves. The inability of cells to process tyrosine can lead to chronic liver damage ending in liver failure, as well as renal disease and rickets. Symptoms such as poor growth and enlarged liver are associated with the clinical presentation of the disease. Clinical manifestation of disease occurs typically within the first two years of life. The severity of the disease is correlated with the timing of onset of symptoms, earlier being more severe. Tyrosinemia type I is an autosomal recessive disorder caused by mutations in both copies of the gene encoding the enzyme fumarylacetoacetate hydrolase (FAH). FAH is a metabolic enzyme that catalyzes the conversion of fumarylacetoacetate to fumarate and acetoacetate. It is expressed primarily in the liver and kidney. Loss of FAH activity results in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitisinone
Nitisinone, sold under the brand name Orfadin among others, is a medication used to slow the effects of hereditary tyrosinemia type 1 (HT-1). Uses Nitisinone is used to treat hereditary tyrosinemia type 1 (HT-1) in patients from all ages, in combination with dietary restriction of tyrosine and phenylalanine. Since its first use for this indication in 1991, it has replaced liver transplantation as the first-line treatment for this ultra rare condition. Adverse effects The most common adverse reactions (>1%) for nitisinone are elevated tyrosine levels, thrombocytopenia, leukopenia, conjunctivitis, corneal opacity, keratitis, photophobia, eye pain, blepharitis, cataracts, granulocytopenia, epistaxis, pruritus, exfoliative dermatitis, dry skin, maculopapular rash and alopecia.has several negative side effects; these include but are not limited to: bloated abdomen, dark urine, abdominal pain, feeling of tiredness or weakness, headache, light-colored stools, loss of appetite, weight ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning ''cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a Hydrophobe, hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is Genetic code, encoded by the Genetic code#Codons, codons UAC and UAU in messenger RNA. Functions Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes and functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Hydroxyphenylpyruvate Dioxygenase
4-Hydroxyphenylpyruvate dioxygenase (HPPD), also known as α-ketoisocaproate dioxygenase (KIC dioxygenase), is an Fe(II)-containing non-heme oxygenase that catalyzes the second reaction in the catabolism of tyrosine - the conversion of 4-hydroxyphenylpyruvate into homogentisate. HPPD also catalyzes the conversion of phenylpyruvate to 2-hydroxyphenylacetate and the conversion of α-ketoisocaproate to β-hydroxy β-methylbutyrate. HPPD is an enzyme that is found in nearly all aerobic forms of life. Enzyme mechanism HPPD is categorized within a class of oxygenase enzymes that usually utilize α-ketoglutarate and diatomic oxygen to oxygenate or oxidize a target molecule. However, HPPD differs from most molecules in this class due to the fact that it does not use α-ketoglutarate, and it only utilizes two substrates while adding both atoms of diatomic oxygen into the product, homogentisate. The HPPD reaction occurs through a NIH shift and involves the oxidative decarboxylation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ochronosis
Ochronosis is a syndrome caused by the accumulation of homogentisic acid in connective tissues. The condition was named after the yellowish (ocher-like) discoloration of the tissue seen on microscopic examination. Macroscopically, though, the affected tissues appear bluish-grey because of a light-scattering phenomenon known as the Tyndall effect. The condition is most often associated with alkaptonuria, but can occur from exogenous administration of phenol complex (chemistry), complexes such as hydroquinone. It was first described by Rudolf Virchow in 1865.Findlay GH, et al. Ochronosis. Clinics in Dermatology 1989;7:28-35 Types The two types of ochronosis are endogenous and exogenous. The endogenous variety is an autosomal-recessive disease, known as alkaptonuria, that is caused by a lack of homogentisate oxidase enzyme.Charlín, R., Barcaui, C. B., Kac, B. K., Soares, D. B., Rabello-Fonseca, R. and Azulay-Abulafia, L. (2008), Hydroquinone-induced exogenous ochronosis: a report ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inborn Error Of Metabolism
Inborn errors of metabolism form a large class of genetic diseases involving congenital disorders of enzyme activities. The majority are due to defects of single genes that code for enzymes that facilitate conversion of various substances (substrate (biochemistry), substrates) into others (Product (chemistry), products). In most of the disorders, problems arise due to accumulation of substances which are toxic or interfere with normal function, or due to the effects of reduced ability to synthesize essential compounds. Inborn errors of metabolism are now often referred to as congenital metabolic diseases or inherited metabolic disorders. To this concept it's possible to include the new term of Enzymopathy. This term was created following the study of Biochemical Processes, Biodynamic Enzymology, a science based on the study of the enzymes and their derivated products. Finally, ''inborn errors of metabolism'' were studied for the first time by British physician Archibald Garrod (1857� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaptonuria
Alkaptonuria is a rare inherited genetic disease which is caused by a mutation in the ''HGD'' gene for the enzyme homogentisate 1,2-dioxygenase (); if a person inherits an abnormal copy from both parents (it is a recessive condition), the body accumulates an intermediate substance called homogentisic acid in the blood and tissues. Homogentisic acid and its oxidized form ''alkapton'' are excreted in the urine, giving it an unusually dark color. The accumulating homogentisic acid causes damage to cartilage (ochronosis, leading to osteoarthritis) and heart valves, as well as precipitating as kidney stones and stones in other organs. Symptoms usually develop in people over 30 years old, although the dark discoloration of the urine is present from birth. Apart from treatment of the complications (such as pain relief and joint replacement for the cartilage damage), the drug nitisinone has been found to suppress homogentisic acid production, and research is ongoing as to whether it can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
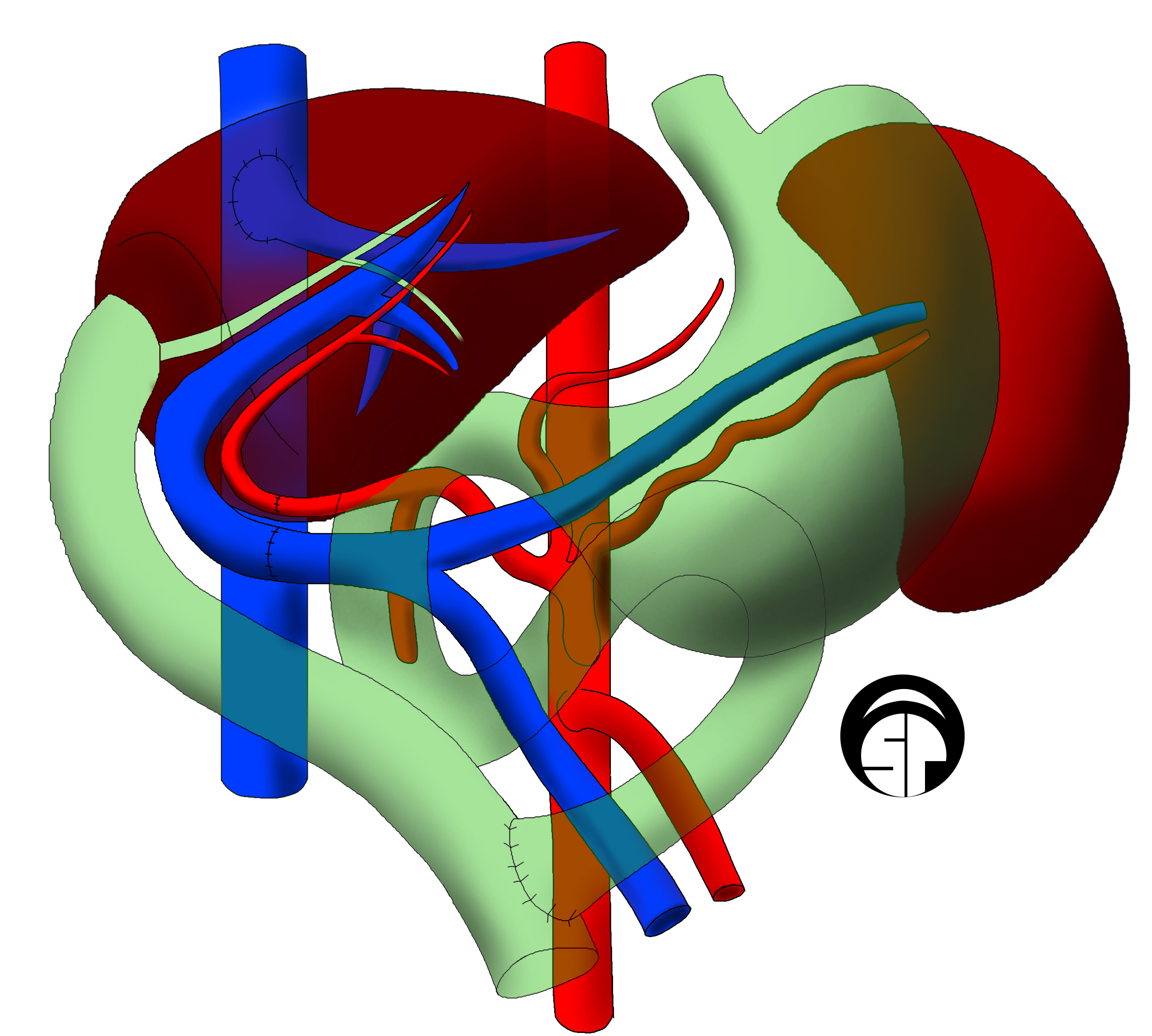
Liver Transplantation
Liver transplantation or hepatic transplantation is the replacement of a diseased liver with the healthy liver from another person (allograft). Liver transplantation is a treatment option for end-stage liver disease and acute liver failure, although availability of donor organs is a major limitation. The most common technique is orthotopic transplantation, in which the native liver is removed and replaced by the donor organ in the same anatomic position as the original liver. The surgical procedure is complex, requiring careful harvest of the donor organ and meticulous implantation into the recipient. Liver transplantation is highly regulated, and only performed at designated transplant medical centers by highly trained transplant physicians and supporting medical team. The duration of the surgery ranges from 4 to 18 hours depending on outcome. Favorable outcomes require careful screening for eligible recipient, as well as a well-calibrated live or cadaveric donor match. Medic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the formula . It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino acid is classified as neutral, and nonpolar because of the inert and hydrophobic nature of the benzyl side chain. The L-isomer is used to biochemically form proteins coded for by DNA. Phenylalanine is a precursor for tyrosine, the monoamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), and the skin pigment melanin. It is encoded by the codons UUU and UUC. Phenylalanine is found naturally in the milk of mammals. It is used in the manufacture of food and drink products and sold as a nutritional supplement for its analgesic and antidepressant effects. It is a direct precursor to the neuromodulator phenethylamine, a commonly used dietary supplement. As an essential amino acid, phenylalanine is n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heredity
Heredity, also called inheritance or biological inheritance, is the passing on of traits from parents to their offspring; either through asexual reproduction or sexual reproduction, the offspring cells or organisms acquire the genetic information of their parents. Through heredity, variations between individuals can accumulate and cause species to evolve by natural selection. The study of heredity in biology is genetics. Overview In humans, eye color is an example of an inherited characteristic: an individual might inherit the "brown-eye trait" from one of the parents. Inherited traits are controlled by genes and the complete set of genes within an organism's genome is called its genotype. The complete set of observable traits of the structure and behavior of an organism is called its phenotype. These traits arise from the interaction of its genotype with the environment. As a result, many aspects of an organism's phenotype are not inherited. For example, suntanned skin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Low-protein Diet
A low-protein diet is a diet in which people decrease their intake of protein. A low-protein diet is used as a therapy for inherited metabolic disorders, such as phenylketonuria and homocystinuria, and can also be used to treat kidney or liver disease. Low protein consumption appears to reduce the risk of bone breakage, presumably through changes in calcium homeostasis. Consequently, there is no uniform definition of what constitutes low-protein, because the amount and composition of protein for an individual with phenylketonuria would differ substantially from one with homocystinuria or tyrosinemia. History By studying the composition of food in the local population in Germany, Carl von Voit established a standard of 118 grams of protein per day. Russell Henry Chittenden showed that less than half that amount was needed to maintain good health. Protein requirement The daily requirement for humans to remain in nitrogen balance is relatively small. The median human adult requi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |