|
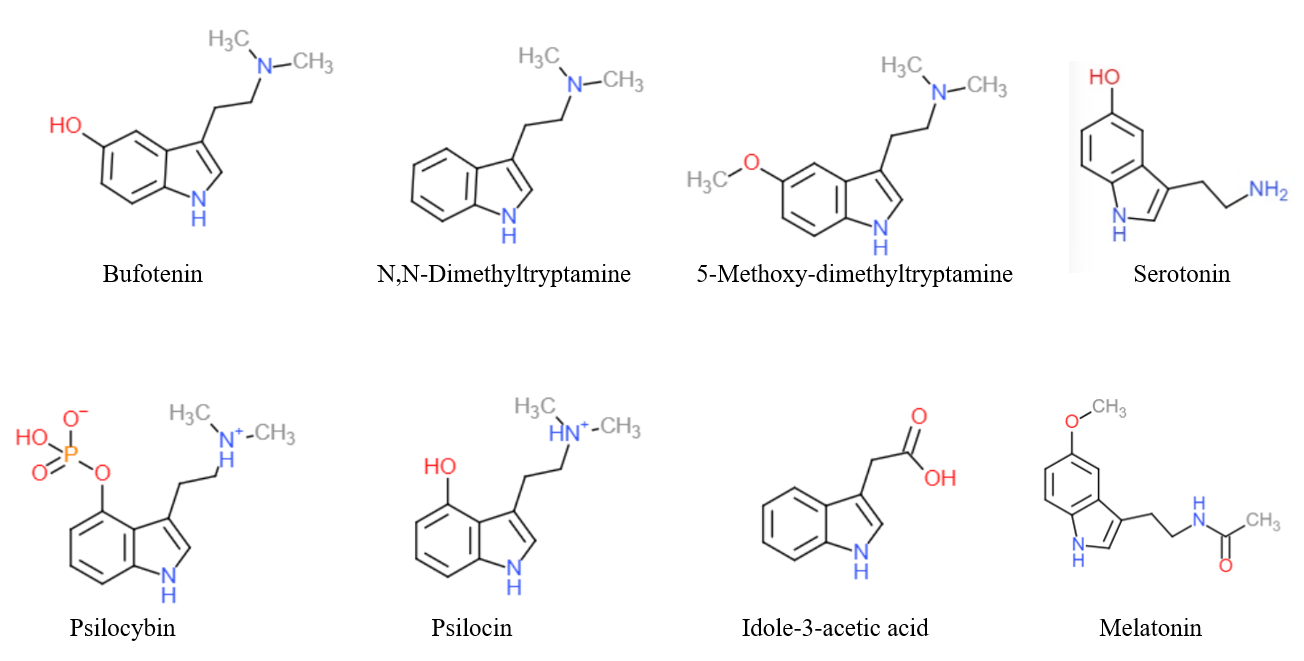
Tryptamines
Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms. Well-known tryptamines include serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine alkaloids are found in fungi, plants and animals; and sometimes used by humans for the neurological or psychotropic effects of the substance. Prominent examples of tryptamine alkaloids include psilocybin (from "psilocybin mushrooms") and DMT. In South America, dimethyltryptamine is obtained from numerous plant sources, like chacruna, and it is often used in ayahuasca brews. Many synthetic tryptamines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptamine Rests General Formula V
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psychedelic Drug
Psychedelics are a subclass of hallucinogenic drugs whose primary effect is to trigger non-ordinary states of consciousness (known as psychedelic experiences or "trips").Pollan, Michael (2018). ''How to Change Your Mind: What the New Science of Psychedelics Teaches Us About Consciousness, Dying, Addiction, Depression, and Transcendence'' Sometimes, they are called classic hallucinogens, serotonergic hallucinogens, or serotonergic psychedelics, and the term ''psychedelics'' is used more broadly to include all hallucinogens; this article uses the narrower definition of ''psychedelics''. Psychedelics cause specific psychological, visual, and auditory changes, and often a substantially altered state of consciousness.Leary, Timothy; Metzner, Ralph (1964). ''The Psychedelic Experience: A Manual Based on The Tibetan Book of the Dead'' Psychedelic states are often compared to meditative, psychodynamic or transcendental types of alterations of mind. The "classical" psychedelics, the psy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alexander Shulgin
Alexander Theodore "Sasha" Shulgin (June 17, 1925 – June 2, 2014) was an American medicinal chemist, biochemist, organic chemist, pharmacologist, psychopharmacologist, and author. He is credited with introducing 3,4-methylenedioxymethamphetamine (MDMA, commonly known as "ecstasy") to psychologists in the late 1970s for psychopharmaceutical use and for the discovery, synthesis and personal bioassay of over 230 psychoactive compounds for their psychedelic and entactogenic potential. In 1991 and 1997, he and his wife Ann Shulgin compiled the books '' PiHKAL'' and ''TiHKAL'' (standing for ''Phenethylamines'' and ''Tryptamines I Have Known And Loved''), from notebooks that extensively described their work and personal experiences with these two classes of psychoactive drugs. Shulgin performed seminal work into the descriptive synthesis of many of these compounds. Some of Shulgin's noteworthy discoveries include compounds of the 2C* family (such as 2C-B) and compounds of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ibogaine
Ibogaine is a naturally occurring psychoactive substance found in plants in the family Apocynaceae such as ''Tabernanthe iboga'', ''Voacanga africana'', and ''Tabernaemontana undulata''. It is a psychedelic with dissociative properties. Preliminary research indicates that it may help counter drug addiction. Its use has been associated with serious side effects and death. Between the years 1990 and 2008, a total of 19 fatalities temporally associated with the ingestion of ibogaine were reported, from which six subjects died of acute heart failure or cardiopulmonary arrest. The total number of subjects who have used it without major side effects during this period remains unknown. It is used as an alternative medicine treatment for drug addiction in some countries. Its prohibition in other countries has slowed scientific research. Ibogaine is also used to facilitate psychological introspection and spiritual exploration. Various derivatives of ibogaine designed to lack psychedel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TiHKAL
''TIHKAL: The Continuation'' is a 1997 book written by Alexander Shulgin and Ann Shulgin about a family of psychoactive drugs known as tryptamines. A sequel to '' PIHKAL: A Chemical Love Story'', ''TIHKAL'' is an acronym that stands for "Tryptamines I Have Known and Loved". Content ''TIHKAL'', much like its predecessor ''PIHKAL'', is divided into two parts. The first part, for which all rights are reserved, begins with a fictionalized autobiography, picking up where the similar section of ''PIHKAL'' left off; it then continues with a collection of essays on topics ranging from psychotherapy and the Jungian mind to the prevalence of DMT in nature, ayahuasca and the War on Drugs. The second part of ''TIHKAL'', which may be conditionally distributed for non-commercial reproduction (see external links An internal link is a type of hyperlink on a web page to another page or resource, such as an image or document, on the same website or domain. Hyperlinks are considered eit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction. Approximately 90% of the serotonin that the body produces is in the intestinal tract. Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin. Serotonin is primarily found in the enteric nervous system located in the gastrointestinal tract (GI tract). However, it is also produced in the central nervous system (CNS), specifically in the raphe nuclei located in the brainstem, Merkel cells located in the skin, pulmonary neuroendocrine cells and taste receptor cells in the tongue. Additionally, serotonin is stored in blood platelets and is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyltryptamine
''N'',''N''-Dimethyltryptamine (DMT or ''N'',''N''-DMT, SPL026) is a substituted tryptamine that occurs in many plants and animals, including human beings, and which is both a derivative and a structural analog of tryptamine. It is used as a psychedelic drug and prepared by various cultures for ritual purposes as an entheogen. DMT has a rapid onset, intense effects, and a relatively short duration of action. For those reasons, DMT was known as the "business trip" during the 1960s in the United States, as a user could access the full depth of a psychedelic experience in considerably less time than with other substances such as LSD or psilocybin mushrooms. DMT can be inhaled, ingested, or injected and its effects depend on the dose, as well as the mode of administration. When inhaled or injected, the effects last a short period of time: about five to 15 minutes. Effects can last three hours or more when orally ingested along with a monoamine oxidase inhibitor (MAOI), such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sumatriptan
Sumatriptan, sold commonly under brand names Imitrex and Treximet among others, is a medication used to treat migraine headaches and cluster headaches. It is taken orally, intranasally, or by subcutaneous injection. Therapeutic effects generally occur within three hours. Its primary effect as a serotonin 5-HT1B/1D receptor agonist can create common side effects such as chest pressure, fatigue, vomiting, tingling, and vertigo. Serious side effects may include serotonin syndrome, heart attacks, strokes, and seizures. With excessive medication overuse headaches may occur. It is unclear if use during pregnancy or breastfeeding is safe. The mechanism of action not entirely clear. It is in the triptan class of medications. Sumatriptan was patented in 1982 and approved for medical use in 1991. It is on the World Health Organization's List of Essential Medicines. It is available as a generic medication. In 2020, it was the 111th most commonly prescribed medication in the United ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psilocybin
Psilocybin ( , ) is a naturally occurring psychedelic prodrug compound produced by more than 200 species of fungi. The most potent are members of the genus ''Psilocybe'', such as '' P. azurescens'', '' P. semilanceata'', and '' P. cyanescens'', but psilocybin has also been isolated from about a dozen other genera. Psilocybin is itself biologically inactive but is quickly converted by the body to psilocin, which has mind-altering effects similar, in some aspects, to those of LSD, mescaline, and DMT. In general, the effects include euphoria, visual and mental hallucinations, changes in perception, a distorted sense of time, and perceived spiritual experiences. It can also cause adverse reactions such as nausea and panic attacks. Imagery found on prehistoric murals and rock paintings of modern-day Spain and Algeria suggests that human usage of psilocybin mushrooms predates recorded history. In Mesoamerica, the mushrooms had long been consumed in spiritual and div ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melatonin
Melatonin is a natural product found in plants and animals. It is primarily known in animals as a hormone released by the pineal gland in the brain at night, and has long been associated with control of the sleep–wake cycle. In vertebrates, melatonin is involved in synchronizing circadian rhythms, including sleep–wake timing and blood pressure regulation, and in control of seasonal rhythmicity including reproduction, fattening, moulting and hibernation. Many of its effects are through activation of the melatonin receptors, while others are due to its role as an antioxidant. In plants, it functions to defend against oxidative stress. It is also present in various foods. Melatonin was discovered in 1958. In addition to its role as a natural hormone, melatonin is used as a dietary supplement and medication in the treatment of sleep disorders such as insomnia and circadian rhythm sleep disorders; for information on melatonin as a supplement and medication, see the melatoni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |