|
Thioredoxin
Thioredoxin (TRX or TXN) is a class of small redox proteins known to be present in all organisms. It plays a role in many important biological processes, including redox signaling. In humans, thioredoxins are encoded by ''TXN'' and ''TXN2'' genes. Loss-of-function mutation of either of the two human thioredoxin genes is lethal at the four-cell stage of the developing embryo. Although not entirely understood, thioredoxin is linked to medicine through their response to reactive oxygen species (ROS). In plants, thioredoxins regulate a spectrum of critical functions, ranging from photosynthesis to growth, flowering and the development and germination of seeds. Thioredoxins play a role in Cell signaling, cell-to-cell communication. Occurrence They are found in nearly all known organisms and are essential for life in mammals. Function The primary function of thioredoxin (Trx) is the reduction of oxidized cysteine residues and the cleavage of disulfide bonds. Multiple in vitro subst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioredoxin Reductase
Thioredoxin reductases (TR, TrxR) () are enzymes that reduce thioredoxin (Trx). Two classes of thioredoxin reductase have been identified: one class in bacteria and some eukaryotes and one in animals. Bacterial TrxR also catalyzes the reduction of glutaredoxin like proteins known as NrdH. Both classes are flavoproteins which function as homodimers. Each monomer contains a flavin adenine dinucleotide, FAD prosthetic group, a NADPH binding domain, and an active site containing a redox-active disulfide bond. Cellular role Thioredoxin reductases are enzymes that catalyze the reduction of thioredoxin and hence they are a central component in the thioredoxin system. Together with thioredoxin (Trx) and NADPH this system's most general description is as a system for reducing disulfide bonds in cells. Electrons are taken from NADPH via TrxR and are transferred to the active site of Trx, which goes on to reduce protein disulfides or other substrates. The Trx system exists in all living ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TXN2
Thioredoxin, mitochondrial also known as thioredoxin-2 is a protein that in humans is encoded by the ''TXN2'' gene on chromosome 22. This nuclear gene encodes a mitochondrial member of the thioredoxin family, a group of small multifunctional redox-active proteins. The encoded protein may play important roles in the regulation of the mitochondrial membrane potential and in protection against oxidant-induced apoptosis. Structure As a thioredoxin, TXN2 is a 12-kDa protein characterized by the redox active site Trp-Cys-Gly-Pro-Cys. In its oxidized (inactive) form, the two cysteines form a disulfide bond. This bond is then reduced by thioredoxin reductase and NADPH to a dithiol, which serves as a disulfide reductase. In contrast to TXN1, TXN2 contains a putative N-terminal mitochondrial targeting sequence, responsible for its mitochondria localization, and lacks structural cysteines. Two mRNA transcripts of the ''TXN2'' gene differ by ~330 bp in the length of the 3′-untranslate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioredoxin Fold
The thioredoxin fold is a protein fold common to enzymes that catalyze disulfide bond formation and isomerization. The fold is named for the canonical example thioredoxin and is found in both prokaryotic and eukaryotic proteins. It is an example of an alpha/beta protein fold that has oxidoreductase activity. The fold's spatial topology consists of a four-stranded antiparallel beta sheet sandwiched between three alpha helices. The strand topology is 2134 with 3 antiparallel to the rest. Sequence conservation Despite sequence variability in many regions of the fold, thioredoxin proteins share a common active site sequence with two reactive cysteine residues: Cys-X-Y-Cys, where X and Y are often but not necessarily hydrophobic amino acids. The reduced form of the protein contains two free thiol In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutaredoxin
Glutaredoxins (also known as Thioltransferase) are small redox enzymes of approximately one hundred amino-acid residues that use glutathione as a cofactor. In humans this oxidation repair enzyme is also known to participate in many cellular functions, including redox signaling and regulation of glucose metabolism. Glutaredoxins are oxidized by substrates, and reduced non-enzymatically by glutathione. In contrast to thioredoxins, which are reduced by thioredoxin reductase, no oxidoreductase exists that specifically reduces glutaredoxins. Instead, glutaredoxins are reduced by the oxidation of glutathione. Reduced glutathione is then regenerated by glutathione reductase. Together these components compose the glutathione system. Like thioredoxin, which functions in a similar way, glutaredoxin possesses an active centre disulfide bond. It exists in either a reduced or an oxidized form where the two cysteine residues are linked in an intramolecular disulfide bond. Glutaredoxins function ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribonucleotide Reductase
Ribonucleotide reductase (RNR), also known as ribonucleoside diphosphate reductase, is an enzyme that catalyzes the formation of deoxyribonucleotides from ribonucleotides. It catalyzes this formation by removing the 2'-hydroxyl group of the ribose ring of nucleoside diphosphates (or triphosphates depending on the class of RNR). This reduction produces deoxyribonucleotides. Deoxyribonucleotides in turn are used in the synthesis of deoxyribonucleic acid, DNA. The reaction catalyzed by RNR is strictly conserved in all living organisms. Furthermore, RNR plays a critical role in regulating the total rate of DNA synthesis so that DNA to cell mass is maintained at a constant ratio during cell division and DNA repair. A somewhat unusual feature of the RNR enzyme is that it catalyzes a reaction that proceeds via a free radical mechanism of action. The substrates for RNR are adenosine diphosphate, ADP, guanosine diphosphate, GDP, cytidine diphosphate, CDP and uridine diphosphate, UDP. dTDP ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ASK1
Apoptosis signal-regulating kinase 1 (ASK1) also known as mitogen-activated protein kinase kinase kinase 5 (MAP3K5) is a member of MAP kinase family and as such a part of mitogen-activated protein kinase pathway. It activates c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinases in a Raf-independent fashion in response to an array of stresses such as oxidative stress, endoplasmic reticulum stress and calcium influx. ASK1 has been found to be involved in cancer, diabetes, rheumatoid arthritis, cardiovascular and neurodegenerative diseases. ''MAP3K5'' gene coding for the protein is located on chromosome 6 at locus 6q22.33. and the transcribed protein contains 1,374 amino acids with 11 kinase subdomains. Northern blot analysis shows that MAP3K5 transcript is abundant in human heart and pancreas. Mechanism of activation Under nonstress conditions ASK1 is oligomerized (a requirement for its activation) through its C-terminal coiled-coil domain (CCC), but remain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Oxygen Species
In chemistry and biology, reactive oxygen species (ROS) are highly Reactivity (chemistry), reactive chemicals formed from diatomic oxygen (), water, and hydrogen peroxide. Some prominent ROS are hydroperoxide (H2O2), superoxide (O2−), hydroxyl radical (OH.), and singlet oxygen(1O2). ROS are pervasive because they are readily produced from O2, which is abundant. ROS are important in many ways, both beneficial and otherwise. ROS function as signals, that turn on and off biological functions. They are intermediates in the redox behavior of O2, which is central to fuel cells. ROS are central to the photodegradation of organic pollutants in the atmosphere. Most often however, ROS are discussed in a biological context, ranging from their effects on aging and their role in causing dangerous genetic mutations. Inventory of ROS ROS are not uniformly defined. All sources include superoxide, singlet oxygen, and hydroxyl radical. Hydrogen peroxide is not nearly as reactive as these s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutathione
Glutathione (GSH, ) is an organic compound with the chemical formula . It is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine. Biosynthesis and occurrence Glutathione biosynthesis involves two adenosine triphosphate-dependent steps: *First, γ-glutamylcysteine is synthesized from L-glutamate and L-cysteine. This conversion requires the enzyme glutamate–cysteine ligase (GCL, glutamate cysteine synthase). This reaction is the rate-limiting step in glutathione synthesis. *Second, glycine is added to the C-terminal of γ-glutamylcysteine. This condensation is ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
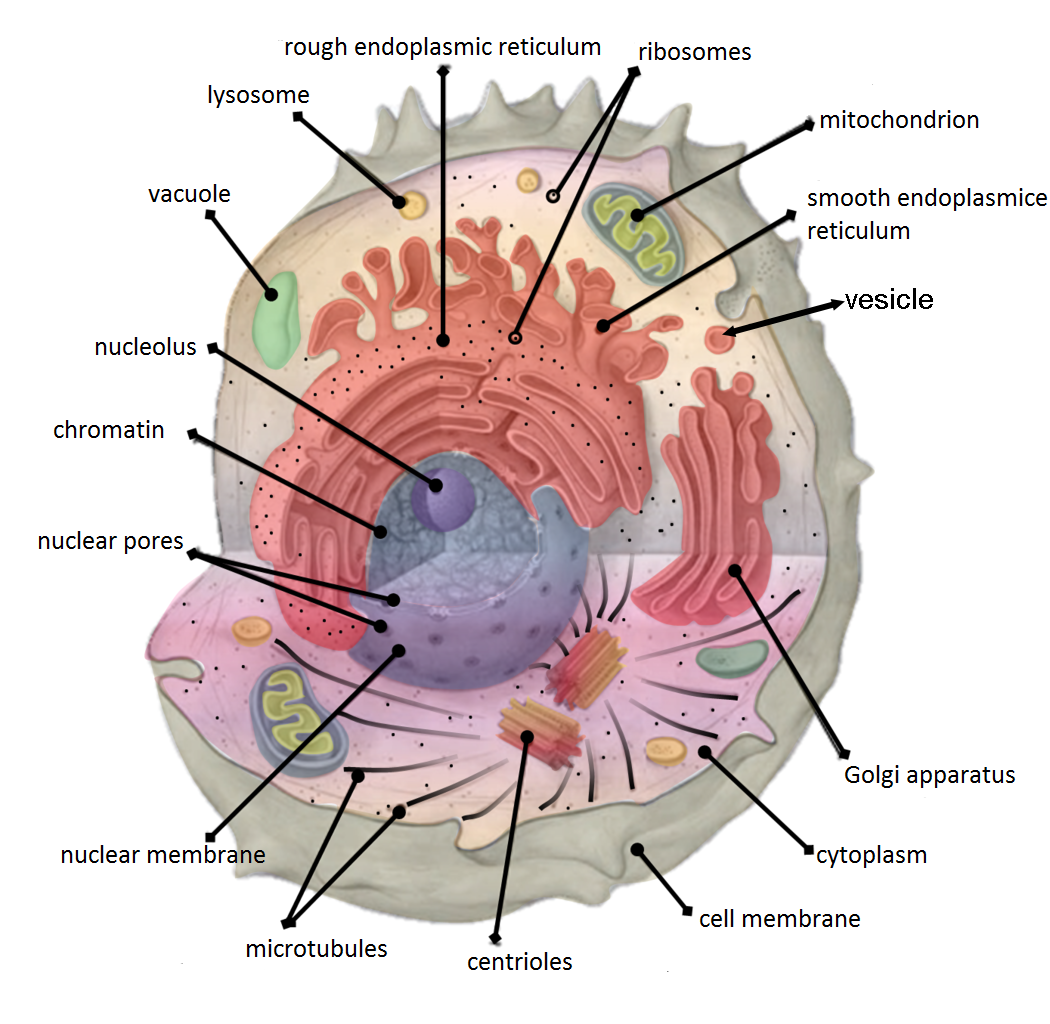
Cell Compartment
Cellular compartments in cell biology comprise all of the closed parts within the cytosol of a eukaryotic cell, usually surrounded by a single or double lipid layer membrane. These compartments are often, but not always, defined as membrane-bound organelles. The formation of cellular compartments is called compartmentalization. Both organelles, the mitochondria and chloroplasts (in photosynthetic organisms), are compartments that are believed to be of endosymbiotic origin. Other compartments such as peroxisomes, lysosomes, the endoplasmic reticulum, the cell nucleus or the Golgi apparatus are not of endosymbiotic origin. Smaller elements like vesicles, and sometimes even microtubules can also be counted as compartments. It was thought that compartmentalization is not found in prokaryotic cells., but the discovery of carboxysomes and many other metabolosomes revealed that prokaryotic cells are capable of making compartmentalized structures, albeit these are in most cases no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plant
Plants are the eukaryotes that form the Kingdom (biology), kingdom Plantae; they are predominantly Photosynthesis, photosynthetic. This means that they obtain their energy from sunlight, using chloroplasts derived from endosymbiosis with cyanobacteria to produce sugars from carbon dioxide and water, using the green pigment chlorophyll. Exceptions are parasitic plants that have lost the genes for chlorophyll and photosynthesis, and obtain their energy from other plants or fungi. Most plants are multicellular organism, multicellular, except for some green algae. Historically, as in Aristotle's biology, the plant kingdom encompassed all living things that were not animals, and included algae and fungi. Definitions have narrowed since then; current definitions exclude fungi and some of the algae. By the definition used in this article, plants form the clade Viridiplantae (green plants), which consists of the green algae and the embryophytes or land plants (hornworts, liverworts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free-radical Theory
The free radical theory of aging states that organisms age because cells accumulate free radical damage over time. A free radical is any atom or molecule that has a single unpaired electron in an outer shell.Erbas M, Sekerci H. "Importance of Free Radicals and Occurring During Food Processing". Serbest Radïkallerïn Onemï Ve Gida Ïsleme Sirasinda Olusumu. 2011: 36(6) 349–56. While a few free radicals such as melanin are not chemically reactive, most biologically relevant free radicals are highly reactive. For most biological structures, free radical damage is closely associated with oxidative damage. Antioxidants are reducing agents, and limit oxidative damage to biological structures by passivating them from free radicals. Strictly speaking, the free radical theory is only concerned with free radicals such as superoxide ( O2− ), but it has since been expanded to encompass oxidative damage from other reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), or perox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Stress
Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal redox state of cells can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA. Oxidative stress from oxidative metabolism causes base damage, as well as strand breaks in DNA. Base damage is mostly indirect and caused by the reactive oxygen species generated, e.g., (superoxide radical), OH ( hydroxyl radical) and (hydrogen peroxide). Further, some reactive oxidative species act as cellular messengers in redox signaling. Thus, oxidative stress can cause disruptions in normal mechanisms of cellular signaling. In humans, oxidative stress is thought to be involved in the development of attention deficit hyperactivity disorder, cancer, Parkin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |