|
Thietane
Thietane is a heterocyclic compound A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ... containing a saturated four-membered ring with three carbon atoms and one sulfur atom. Thietane, and its derivative 2-propylthietane, are strong-smelling mouse alarm pheromones and predator scent analogues. Both the mouse and human olfactory receptors MOR244-3 and OR2T11, respectively, were found to respond to thietane in the presence of copper. References Sulfur heterocycles Four-membered rings Foul-smelling chemicals {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithietane
Dithietanes are saturated heterocyclic compounds that contain two divalent sulfur atoms and two sp3-hybridized carbon centers. Two isomers are possible for this class of organosulfur compounds: 1,2-Dithietanes 1,2-dithietanes, 4-membered rings where the two sulfur atoms are adjacent, are very rare. The first stable 1,2-dithietane to be reported was the so-called dithiatopazine formed by intramolecular photodimerization of a dithiocarbonyl compound. 1,2-Dithietanes are to be distinguished from 1,2-dithietes, containing two adjacent sulfur atoms and two sp2-hybridized carbon centers. A stable 1,2-dithietane ''derivative'' is ''trans''-3,4-diethyl-1,2-dithietane 1,1-dioxide, formed by the spontaneous dimerization of the lachrymatory agent syn-propanethial-S-oxide, found in onion. 1,3-Dithietanes In 1,3-dithietanes, the sulfur atoms are non-adjacent. 1,3-Dithietane itself, a colorless, easily sublimed, crystalline, unpleasant-smelling solid with melting point 105-106&nb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different chemical element, elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiirane
Thiirane, more commonly known as ethylene sulfide, is the cyclic chemical compound with the formula C2H4S. It is the smallest sulfur-containing heterocycle and the simplest episulfide. Like many organosulfur compounds, this species has a highly unpleasant odour. Thiirane is also used to describe any derivative of the parent ethylene sulfide. Structure According to electron diffraction Electron diffraction refers to the bending of electron beams around atomic structures. This behaviour, typical for Wave (physics), waves, is applicable to electrons due to the wave–particle duality stating that electrons behave as both particle ..., the C-C and C-S distances in ethylene sulfide are respectively 1.473 and 1.811 Å. The C-C-S and C-S-C angles are respectively 66.0 and 48.0°. Preparation and reactions It can be prepared by the reaction of ethylene carbonate and KSCN. For this purpose the KSCN is first melted under vacuum to remove water. :KSCN + C2H4O2CO → KOCN + C2H4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Heterocycles
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Today, almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere The greatest commercial use of the element is the production of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
OR2T11
Olfactory receptor 2T11 is a protein that in humans is encoded by the ''OR2T11'' gene. Olfactory receptors interact with odorant molecules in the nose, to initiate a neuronal response that triggers the perception of a smell. The olfactory receptor proteins are members of a large family of G-protein-coupled receptors (GPCR) arising from single coding-exon genes. Olfactory receptors share a 7-transmembrane domain structure with many neurotransmitters and hormone receptors and are responsible for the recognition and G protein-mediated transduction of odorant signals. The olfactory receptor gene family is the largest in the genome. The nomenclature assigned to the olfactory receptor genes and proteins for this organism is independent of other organisms. Ligands * tert-Butylthiol (the response is enhanced by the presence of ionic copper). * Ethanethiol (the response is enhanced by the presence of ionic copper). * 2-Propenethiol ( allyl mercaptan) (the response is enhanced by the p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Olfactory Receptors
Olfactory receptors (ORs), also known as odorant receptors, are chemoreceptors expressed in the cell membranes of olfactory receptor neurons and are responsible for the detection of odorants (for example, compounds that have an odor) which give rise to the sense of smell. Activated olfactory receptors trigger nerve impulses which transmit information about odor to the brain. These receptors are members of the class A rhodopsin-like family of G protein-coupled receptors (GPCRs). The olfactory receptors form a multigene family consisting of around 800 genes in humans and 1400 genes in mice. Expression In vertebrates, the olfactory receptors are located in both the cilia and synapses of the olfactory sensory neurons and in the epithelium of the human airway. In insects, olfactory receptors are located on the antennae and other chemosensory organs. Sperm cells also express odor receptors, which are thought to be involved in chemotaxis to find the egg cell. Mechanism Ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pheromones
A pheromone () is a secreted or excreted chemical factor that triggers a social response in members of the same species. Pheromones are chemicals capable of acting like hormones outside the body of the secreting individual, to affect the behavior of the receiving individuals. There are '' alarm pheromones'', ''food trail pheromones'', '' sex pheromones'', and many others that affect behavior or physiology. Pheromones are used by many organisms, from basic unicellular prokaryotes to complex multicellular eukaryotes. Their use among insects has been particularly well documented. In addition, some vertebrates, plants and ciliates communicate by using pheromones. The ecological functions and evolution of pheromones are a major topic of research in the field of chemical ecology. Background The portmanteau word "pheromone" was coined by Peter Karlson and Martin Lüscher in 1959, based on the Greek φερω ''pheroo'' ('I carry') and ὁρμων ''hormon'' ('stimulating'). Pher ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alarm
An alarm device is a mechanism that gives an audible, visual or other kind of alarm signal to alert someone to a problem or condition that requires urgent attention. Alphabetical musical instruments Etymology The word ''alarm'' comes from the Old French ''a l'arme'' meaning "to the arms", or "to the weapons", telling armed men to pick up their weapons and get ready for action because an enemy may have suddenly appeared. The word ''alarum'' is an archaic form of ''alarm''. It was sometimes used as a call to arms in the stage directions of Elizabethan dramas. The term comes from the Italian ''all'armi'' and appears 89 times in Shakespeare's first folio. Often explained as the off-stage sounds of conflict or disturbance, recent research suggests a bell or drum may have been used to rouse soldiers from sleep. History and development Early alarm devices were often bells, drums, other musical instruments, or any items which made unusual loud noises that attracted the attent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturated And Unsaturated Compounds
In chemistry, a saturated compound is a chemical compound (or ion) that resists the addition reactions, such as hydrogenation, oxidative addition, and binding of a Lewis base. The term is used in many contexts and for many classes of chemical compounds. Overall, saturated compounds are less reactive than unsaturated compounds. Saturation is derived from the Latin word ''saturare'', meaning 'to fill'. Organic chemistry Unsaturated compounds generally carry out typical addition reactions that are not possible with saturated compounds such as alkanes. A saturated organic compound has only single bonds between carbon atoms. An important class of saturated compounds are the alkanes. Many saturated compounds have functional groups, e.g., alcohols. Unsaturated organic compounds The concept of saturation can be described using various naming systems, formulas, and analytical tests. For instance, IUPAC nomenclature is a system of naming conventions used to describe the type an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
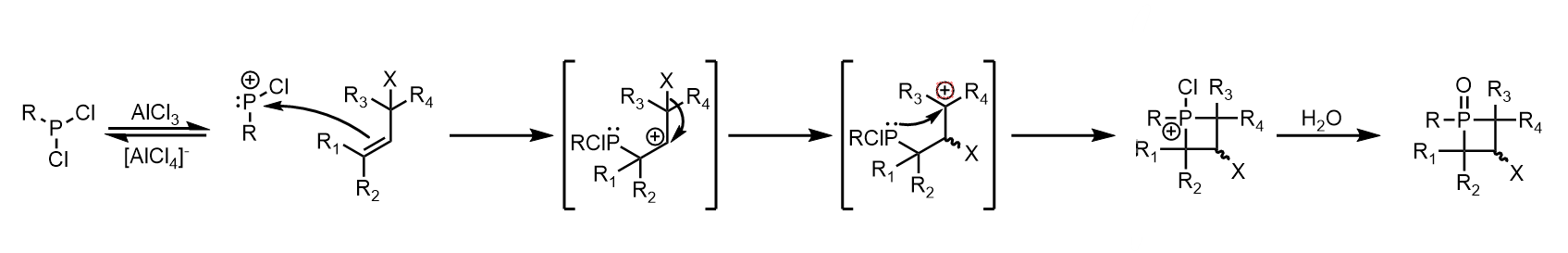
Phosphetane
A phosphetane is a 4-membered organophosphorus heterocycle. The parent phosphetane molecule, which has the formula C3H7P, is one atom larger than phosphiranes, one smaller than phospholes, and is the heavy-atom analogue of azetidines. The first known phosphetane synthesis was reported in 1957 by Kosolapoff and Struck, but the method was both inefficient and hard to reproduce, with yields rarely exceeding 1%. A far more efficient method was reported in 1962 by McBride, whose method allowed for the first studies into the physical and chemical properties of phosphetanes. Phosphetanes are a well understood class of molecules that have found broad applications as chemical building blocks, reagents for organic/inorganic synthesis, and ligands in coordination chemistry. Synthesis Many methods towards the synthesis of phosphetanes have been developed since 1957. The following are the most utilized. McBride method (Electrophilic addition to olefins) The method initially outlined by McB ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azetidine
Azetidine is a Saturated and unsaturated compounds, saturated heterocyclic organic compound containing three carbon atoms and one nitrogen atom. It is a liquid at room temperature with a strong odor of ammonia and is strongly basic compared to most secondary amines. Synthesis and occurrence Azetidines can be prepared by reduction of azetidinones (β-lactams) with lithium aluminium hydride. Even more effective is a mixture of lithium aluminium hydride and aluminium trichloride, a source of "AlClH2" and "AlCl2H". Azetidine can also be produced by a multistep route from 3-Amino-1-propanol, 3-amino-1-propanol. Regio- and diastereoselective synthesis of 2-arylazetidines could be performed from appropriately substituted oxiranes via ring transformation. It is controlled by Baldwin's Rules with remarkable functional group tolerance. Azetidine and its derivatives are relatively rare structural motifs in natural products. They are a component of mugineic acids and penaresidins. Perhaps ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxetane
Oxetane, or 1,3-propylene oxide, is a heterocyclic organic compound with the molecular formula , having a four-membered ring with three carbon atoms and one oxygen atom. The term "an oxetane" or "oxetanes" refer to any organic compound containing the oxetane ring. Production A typical well-known method of preparation is the reaction of potassium hydroxide with 3-chloropropyl acetate at 150 °C: : Yield of oxetane made this way is c. 40%, as the synthesis can lead to a variety of by-products. Another possible reaction to form an oxetane ring is the Paternò–Büchi reaction. The oxetane ring can also be formed through diol cyclization as well as through decarboxylation of a six-membered cyclic carbonate. Taxol Paclitaxel (Taxol) is an example of a natural product containing an oxetane ring. Taxol has become a major point of interest among researchers due to its unusual structure and success in the involvement of cancer treatment. The attached oxetane ring is an imp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |