|
Thermoelectrics
Thermoelectric materials show the thermoelectric effect in a strong or convenient form. The ''thermoelectric effect'' refers to phenomena by which either a temperature difference creates an electric potential or an electric current creates a temperature difference. These phenomena are known more specifically as the Seebeck effect (creating a voltage from temperature difference), Peltier effect (driving heat flow with an electric current), and Thomson effect (reversible heating or cooling within a conductor when there is both an electric current and a temperature gradient). While all materials have a nonzero thermoelectric effect, in most materials it is too small to be useful. However, low-cost materials that have a sufficiently strong thermoelectric effect (and other required properties) are also considered for applications including power generation and refrigeration. The most commonly used thermoelectric material is based on bismuth telluride (). Thermoelectric materials are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermogenerator
A thermoelectric generator (TEG), also called a Seebeck generator, is a solid state device that converts heat flux (temperature differences) directly into electrical energy through a phenomenon called the ''Seebeck effect'' (a form of thermoelectric effect). Thermoelectric generators function like heat engines, but are less bulky and have no moving parts. However, TEGs are typically more expensive and less efficient. Thermoelectric generators could be used in power plants to convert waste heat into additional electrical power and in automobiles as automotive thermoelectric generators (ATGs) to increase fuel efficiency. Radioisotope thermoelectric generators use radioisotopes to generate the required temperature difference to power space probes. History In 1821, Thomas Johann Seebeck discovered that a thermal gradient formed between two different conducting material (has electromagnetic property) can produce electricity. At the heart of the thermoelectric effect is the fact tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
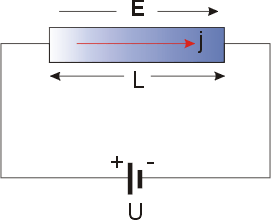
Seebeck Coefficient
The Seebeck coefficient (also known as thermopower, thermoelectric power, and thermoelectric sensitivity) of a material is a measure of the magnitude of an induced thermoelectric voltage in response to a temperature difference across that material, as induced by the Seebeck effect. The SI unit of the Seebeck coefficient is volts per kelvin (V/K), although it is more often given in microvolts per kelvin (μV/K). The use of materials with a high Seebeck coefficient is one of many important factors for the efficient behaviour of thermoelectric generators and thermoelectric coolers. More information about high-performance thermoelectric materials can be found in the Thermoelectric materials article. In thermocouples the Seebeck effect is used to measure temperatures, and for accuracy it is desirable to use materials with a Seebeck coefficient that is stable over time. Physically, the magnitude and sign of the Seebeck coefficient can be approximately understood as being given by the e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa. Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal conductivity. For instance, metals typically have high thermal conductivity and are very efficient at conducting heat, while the opposite is true for insulating materials like Rockwool or Styrofoam. Correspondingly, materials of high thermal conductivity are widely used in heat sink applications, and materials of low thermal conductivity are used as thermal insulation. The reciprocal of thermal conductivity is called thermal resistivity. The defining equation for thermal conductivity is \mathbf = - k \nabla T, where \mathbf is the heat flux, k is the thermal conductivity, and \nabla T is the temperature gradient. This is known as Fourier's Law for heat conduction. Although commonly expressed as a scalar, the most general form of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wiedemann–Franz Law
In physics, the Wiedemann–Franz law states that the ratio of the electronic contribution of the thermal conductivity (''κ'') to the electrical conductivity (''σ'') of a metal is proportional to the temperature (''T''). : \frac \kappa \sigma = LT Theoretically, the proportionality constant ''L'', known as the Lorenz number, is equal to : L = \frac \kappa = \frac 3 \left(\frac e \right)^2 = 2.44\times 10^\;\mathrm^2\mathrm^, where ''k''B is Boltzmann's constant and ''e'' is the elementary charge. This empirical law is named after Gustav Wiedemann and Rudolph Franz, who in 1853 reported that ''κ''/''σ'' has approximately the same value for different metals at the same temperature. The proportionality of ''κ''/''σ'' with temperature was discovered by Ludvig Lorenz in 1872. Derivation Qualitatively, this relationship is based upon the fact that the heat and electrical transport both involve the free electrons in the metal. The mathematical e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoelectric Effect
The thermoelectric effect is the direct conversion of temperature differences to electric voltage and vice versa via a thermocouple. A thermoelectric device creates a voltage when there is a different temperature on each side. Conversely, when a voltage is applied to it, heat is transferred from one side to the other, creating a temperature difference. At the atomic scale, an applied temperature gradient causes charge carriers in the material to diffuse from the hot side to the cold side. This effect can be used to generate electricity, measure temperature or change the temperature of objects. Because the direction of heating and cooling is affected by the applied voltage, thermoelectric devices can be used as temperature controllers. The term "thermoelectric effect" encompasses three separately identified effects: the Seebeck effect, Peltier effect, and Thomson effect. The Seebeck and Peltier effects are different manifestations of the same physical process; textbooks may re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phonons
In physics, a phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, specifically in solids and some liquids. A type of quasiparticle, a phonon is an excited state in the quantum mechanical quantization of the modes of vibrations for elastic structures of interacting particles. Phonons can be thought of as quantized sound waves, similar to photons as quantized light waves. The study of phonons is an important part of condensed matter physics. They play a major role in many of the physical properties of condensed matter systems, such as thermal conductivity and electrical conductivity, as well as in models of neutron scattering and related effects. The concept of phonons was introduced in 1932 by Soviet physicist Igor Tamm. The name ''phonon'' comes from the Greek word (), which translates to ''sound'' or ''voice'', because long-wavelength phonons give rise to sound. The name is analogous to the word ''photon''. Definiti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermi Energy
The Fermi energy is a concept in quantum mechanics usually referring to the energy difference between the highest and lowest occupied single-particle states in a quantum system of non-interacting fermions at absolute zero temperature. In a Fermi gas, the lowest occupied state is taken to have zero kinetic energy, whereas in a metal, the lowest occupied state is typically taken to mean the bottom of the conduction band. The term "Fermi energy" is often used to refer to a different yet closely related concept, the Fermi ''level'' (also called electrochemical potential).The use of the term "Fermi energy" as synonymous with Fermi level (a.k.a. electrochemical potential) is widespread in semiconductor physics. For example:''Electronics (fundamentals And Applications)''by D. Chattopadhyay''Semiconductor Physics and Applications''by Balkanski and Wallis. There are a few key differences between the Fermi level and Fermi energy, at least as they are used in this article: * The Fermi energy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conduction Band
In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level, and thus determine the electrical conductivity of the solid. In nonmetals, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature, while the conduction band is the lowest range of vacant electronic states. On a graph of the electronic band structure of a material, the valence band is located below the Fermi level, while the conduction band is located above it. The distinction between the valence and conduction bands is meaningless in metals, because conduction occurs in one or more partially filled bands that take on the properties of both the valence and conduction bands. Band gap In semiconductors and insulators the two bands are separated by a band gap, while in semimetals the bands overlap. A band gap is an energy range in a solid where no electron states can exist due to the quantization of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron's mass is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum ( spin) of a half-integer value, expressed in units of the reduced Planck constant, . Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: They can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phonon
In physics, a phonon is a collective excitation in a periodic, Elasticity (physics), elastic arrangement of atoms or molecules in condensed matter physics, condensed matter, specifically in solids and some liquids. A type of quasiparticle, a phonon is an excited state in the quantum mechanical Quantization (physics), quantization of the mode of vibration, modes of vibrations for elastic structures of interacting particles. Phonons can be thought of as quantized sound waves, similar to photons as quantized light waves. The study of phonons is an important part of condensed matter physics. They play a major role in many of the physical properties of condensed matter systems, such as thermal conductivity and electrical conductivity, as well as in models of neutron scattering and related effects. The concept of phonons was introduced in 1932 by Soviet Union, Soviet physicist Igor Tamm. The name ''phonon'' comes from the Ancient Greek language, Greek word (), which translates to ''so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Band Structure
In solid-state physics, the electronic band structure (or simply band structure) of a solid describes the range of energy levels that electrons may have within it, as well as the ranges of energy that they may not have (called ''band gaps'' or ''forbidden bands''). Band theory derives these bands and band gaps by examining the allowed quantum mechanical wave functions for an electron in a large, periodic lattice of atoms or molecules. Band theory has been successfully used to explain many physical properties of solids, such as electrical resistivity and optical absorption, and forms the foundation of the understanding of all solid-state devices (transistors, solar cells, etc.). Why bands and band gaps occur The electrons of a single, isolated atom occupy atomic orbitals each of which has a discrete energy level. When two or more atoms join together to form a molecule, their atomic orbitals overlap and hybridize. Similarly, if a large number ''N'' of identical atoms come ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and "rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third category of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |