|
Thermodynamic Potentials
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantities but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to various topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering, and mechanical engineering, as well as other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to formulate a concise defi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physics
Physics is the scientific study of matter, its Elementary particle, fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which relates to the order of nature, or, in other words, to the regular succession of events." It is one of the most fundamental scientific disciplines. "Physics is one of the most fundamental of the sciences. Scientists of all disciplines use the ideas of physics, including chemists who study the structure of molecules, paleontologists who try to reconstruct how dinosaurs walked, and climatologists who study how human activities affect the atmosphere and oceans. Physics is also the foundation of all engineering and technology. No engineer could design a flat-screen TV, an interplanetary spacecraft, or even a better mousetrap without first understanding the basic laws of physics. (...) You will come to see physics as a towering achievement of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanical Engineering
Mechanical engineering is the study of physical machines and mechanism (engineering), mechanisms that may involve force and movement. It is an engineering branch that combines engineering physics and engineering mathematics, mathematics principles with materials science, to design, analyze, manufacture, and maintain mechanical systems. It is one of the oldest and broadest of the List of engineering branches, engineering branches. Mechanical engineering requires an understanding of core areas including mechanics, Analytical dynamics, dynamics, thermodynamics, materials science, design, structural analysis, and electricity. In addition to these core principles, mechanical engineers use tools such as computer-aided design (CAD), computer-aided manufacturing (CAM), computer-aided engineering (CAE), and product lifecycle management to design and analyze manufacturing plants, industrial equipment and industrial machinery, machinery, HVAC, heating and cooling systems, transport systems, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Thermodynamics
Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the ''spontaneity'' of processes. The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the "fundamental equations of Gibbs" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics. History In 1865, the German physicist Rudolf Clausius, in his ''Mechanical Theory of Heat'', suggested that the principles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanical Heat Engine
A heat engine is a system that transfers thermal energy to do work (physics), mechanical or voltage#Definition, electrical work. While originally conceived in the context of mechanical energy, the concept of the heat engine has been applied to various other kinds of energy, particularly electrical energy, electrical, since at least the late 19th century. The heat engine does this by bringing a working substance from a higher state temperature to a lower state temperature. A heat source generates thermal energy that brings the working substance to the higher temperature state. The working substance generates work in the thermodynamic system, working body of the engine while heat transfer, transferring heat to the colder thermal reservoir, sink until it reaches a lower temperature state. During this process some of the thermal energy is converted into energy, work by exploiting the properties of the working substance. The working substance can be any system with a non-zero heat ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Virial Theorem
In mechanics, the virial theorem provides a general equation that relates the average over time of the total kinetic energy of a stable system of discrete particles, bound by a conservative force (where the work done is independent of path), with that of the total potential energy of the system. Mathematically, the theorem states that \langle T \rangle = -\frac12\,\sum_^N \langle\mathbf_k \cdot \mathbf_k\rangle, where T is the total kinetic energy of the N particles, F_k represents the force on the kth particle, which is located at position , and angle brackets represent the average over time of the enclosed quantity. The word virial for the right-hand side of the equation derives from , the Latin word for "force" or "energy", and was given its technical definition by Rudolf Clausius in 1870. The significance of the virial theorem is that it allows the average total kinetic energy to be calculated even for very complicated systems that defy an exact solution, such as those co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Second Law Of Thermodynamics
The second law of thermodynamics is a physical law based on Universal (metaphysics), universal empirical observation concerning heat and Energy transformation, energy interconversions. A simple statement of the law is that heat always flows spontaneously from hotter to colder regions of matter (or 'downhill' in terms of the temperature gradient). Another statement is: "Not all heat can be converted into Work (thermodynamics), work in a cyclic process."Young, H. D; Freedman, R. A. (2004). ''University Physics'', 11th edition. Pearson. p. 764. The second law of thermodynamics establishes the concept of entropy as a physical property of a thermodynamic system. It predicts whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. For example, the first law allows the process of a cup falling off a table and breaking on the floor, as well as allowi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theory Of Heat
The history of thermodynamics is a fundamental strand in the history of physics, the history of chemistry, and the history of science in general. Due to the relevance of thermodynamics in much of science and technology, its history is finely woven with the developments of classical mechanics, quantum mechanics, magnetism, and chemical kinetics, to more distant applied fields such as meteorology, information theory, and biology (physiology), and to technology, technological developments such as the steam engine, internal combustion engine, cryogenics and electricity generation. The development of thermodynamics both drove and was driven by atomic theory. It also, albeit in a subtle manner, motivated new directions in probability and statistics; see, for example, the timeline of thermodynamics. Antiquity The ancients viewed heat as that related to fire. In 3000 BC, the ancient Egyptians viewed heat as related to origin mythologies. The ancient Indian philosophy including ''Vedic p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carnot Cycle
A Carnot cycle is an ideal thermodynamic cycle proposed by French physicist Nicolas Léonard Sadi Carnot, Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. By Carnot's theorem (thermodynamics), Carnot's theorem, it provides an upper limit on the Thermal efficiency, efficiency of any classical Heat engine, thermodynamic engine during the conversion of heat into Work (thermodynamics), work, or conversely, the efficiency of a refrigeration system in creating a temperature difference through the application of work to the system. In a Carnot cycle, a Thermodynamic system, system or engine transfers energy in the form of heat between two thermal reservoirs at temperatures T_H and T_C (referred to as the hot and cold reservoirs, respectively), and a part of this transferred energy is converted to the work done by the system. The cycle is Reversible process (thermodynamics), reversible, and entropy is Conserved quantity, conserved, merely transferred between the th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rudolf Clausius
Rudolf Julius Emanuel Clausius (; 2 January 1822 – 24 August 1888) was a German physicist and mathematician and is considered one of the central founding fathers of the science of thermodynamics. By his restatement of Sadi Carnot's principle known as the Carnot cycle, he gave the theory of heat a truer and sounder basis. His most important paper, "On the Moving Force of Heat", published in 1850, first stated the basic ideas of the second law of thermodynamics. In 1865 he introduced the concept of entropy. In 1870 he introduced the virial theorem, which applied to heat. Life Clausius was born in Köslin (now Koszalin, Poland) in the Province of Pomerania in Prussia. His father was a Protestant pastor and school inspector, and Rudolf studied in the school of his father. In 1838, he went to the Gymnasium in Stettin. Clausius graduated from the University of Berlin in 1844 where he had studied mathematics and physics since 1840 with, among others, Gustav Magnus, Peter Gusta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
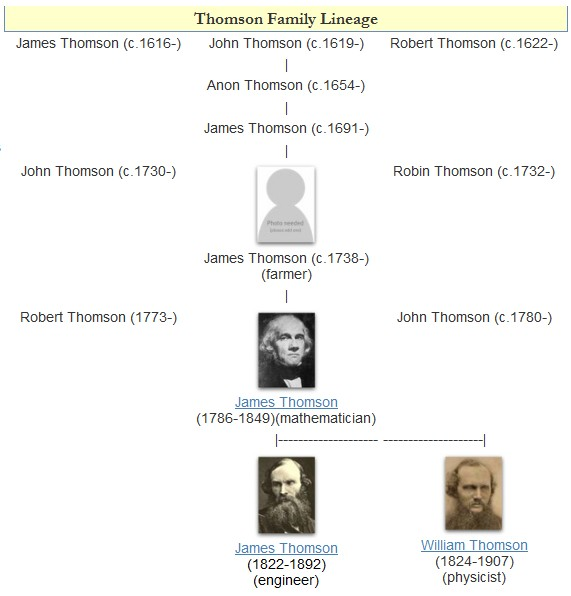
William Thomson, 1st Baron Kelvin
William Thomson, 1st Baron Kelvin (26 June 182417 December 1907), was a British mathematician, Mathematical physics, mathematical physicist and engineer. Born in Belfast, he was the Professor of Natural Philosophy (Glasgow), professor of Natural Philosophy at the University of Glasgow for 53 years, where he undertook significant research on the mathematical analysis of electricity, was instrumental in the formulation of the first and second laws of thermodynamics, and contributed significantly to unifying physics, which was then in its infancy of development as an emerging academic discipline. He received the Royal Society's Copley Medal in 1883 and served as its President of the Royal Society, president from 1890 to 1895. In 1892, he became the first scientist to be elevated to the House of Lords. Absolute temperatures are stated in units of kelvin in Lord Kelvin's honour. While the existence of a coldest possible temperature, absolute zero, was known before his work, Kelvin d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Napoleonic Wars
{{Infobox military conflict , conflict = Napoleonic Wars , partof = the French Revolutionary and Napoleonic Wars , image = Napoleonic Wars (revision).jpg , caption = Left to right, top to bottom:Battles of Battle of Austerlitz, Austerlitz, Fall of Berlin (1806), Berlin, Battle of Friedland, Friedland, Battle of Aspern-Essling, Aspern-Essling, French occupation of Moscow, Moscow, Battle of Leipzig, Leipzig and Battle of Paris (1814), Paris , date = {{start and end dates, 1803, 5, 18, 1815, 11, 20, df=yes({{Age in years, months, weeks and days, month1=05, day1=18, year1=1803, month2=11, day2=20, year2=1815) , place = Atlantic Ocean, Caucasus, Europe, French Guiana, Mediterranean Sea, North Sea, West Indies, Ottoman Egypt, Egypt, East Indies. , result = Coalition victory , combatant1 = Coalition forces of the Napoleonic Wars, Coalition forces:{{flagcountry, United Kingdom of Great Britain and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicolas Léonard Sadi Carnot
Nicolas Léonard Sadi Carnot (; 1 June 1796 – 24 August 1832) was a French people, French military engineering, military engineer and physicist. A graduate of the École polytechnique, Carnot served as an officer in the Engineering Arm (''le génie'') of the French Army. He also pursued scientific studies and in June 1824 published an essay titled ''Reflections on the Motive Power of Fire''. In that book, which would be his only publication, Carnot developed the first successful theory of the Thermal efficiency, maximum efficiency of heat engines. Carnot's scientific work attracted little attention during his lifetime, but in 1834 it became the object of a detailed commentary and explanation by another French engineer, Émile Clapeyron. Clapeyron's commentary in turn attracted the attention of William Thomson, 1st Baron Kelvin, William Thomson (later Lord Kelvin) and Rudolf Clausius. Thomson used Carnot's analysis to develop an absolute thermodynamic temperature scale, whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |