|
Thermodynamic Operation
A thermodynamic operation is an externally imposed manipulation that affects a thermodynamic system. The change can be either in the connection or wall between a thermodynamic system and its surroundings, or in the value of some variable in the surroundings that is in contact with a wall of the system that allows transfer of the extensive quantity belonging that variable.Giles, R. (1964), p. 22.Lieb, E.H., Yngvason, J. (1999). It is assumed in thermodynamics that the operation is conducted in ignorance of any pertinent microscopic information. A thermodynamic operation requires a contribution from an independent external agency, that does not come from the passive properties of the systems. Perhaps the first expression of the distinction between a thermodynamic operation and a thermodynamic process is in Kelvin's statement of the second law of thermodynamics: "It is impossible, by means of inanimate material agency, to derive mechanical effect from any portion of matter by cooling i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic System
A thermodynamic system is a body of matter and/or radiation separate from its surroundings that can be studied using the laws of thermodynamics. Thermodynamic systems can be passive and active according to internal processes. According to internal processes, passive systems and active systems are distinguished: passive, in which there is a redistribution of available energy, active, in which one type of energy is converted into another. Depending on its interaction with the environment, a thermodynamic system may be an isolated system, a Closed system#In thermodynamics, closed system, or an Open system (systems theory), open system. An isolated system does not exchange matter or energy with its surroundings. A closed system may exchange heat, experience forces, and exert forces, but does not exchange matter. An open system can interact with its surroundings by exchanging both matter and energy. The physical condition of a thermodynamic system at a given time is described by its ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carnot Heat Engine
A Carnot heat engine is a theoretical heat engine that operates on the Carnot cycle. The basic model for this engine was developed by Nicolas Léonard Sadi Carnot in 1824. The Carnot engine model was graphically expanded by Benoît Paul Émile Clapeyron in 1834 and mathematically explored by Rudolf Clausius in 1857, work that led to the fundamental thermodynamic concept of entropy. The Carnot engine is the most efficient heat engine which is theoretically possible. The efficiency depends only upon the absolute temperatures of the hot and cold heat reservoirs between which it operates. A heat engine acts by transferring energy from a warm region to a cool region of space and, in the process, converting some of that energy to mechanical work. The cycle may also be reversed. The system may be worked upon by an external force, and in the process, it can transfer thermal energy from a cooler system to a warmer one, thereby acting as a refrigerator or heat pump rather than a heat en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Poincaré Recurrence Theorem
In mathematics and physics, the Poincaré recurrence theorem states that certain dynamical systems will, after a sufficiently long but finite time, return to a state arbitrarily close to (for continuous state systems), or exactly the same as (for discrete state systems), their initial state. The Poincaré recurrence time is the length of time elapsed until the recurrence. This time may vary greatly depending on the exact initial state and required degree of closeness. The result applies to isolated mechanical systems subject to some constraints, e.g., all particles must be bound to a finite volume. The theorem is commonly discussed in the context of ergodic theory, dynamical systems and statistical mechanics. Systems to which the Poincaré recurrence theorem applies are called conservative systems. The theorem is named after Henri Poincaré, who discussed it in 1890. A proof was presented by Constantin Carathéodory using measure theory in 1919. Precise formulation Any dynam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homogeneous Function
In mathematics, a homogeneous function is a function of several variables such that the following holds: If each of the function's arguments is multiplied by the same scalar (mathematics), scalar, then the function's value is multiplied by some power of this scalar; the power is called the degree of homogeneity, or simply the ''degree''. That is, if is an integer, a function of variables is homogeneous of degree if :f(sx_1,\ldots, sx_n)=s^k f(x_1,\ldots, x_n) for every x_1, \ldots, x_n, and s\ne 0. This is also referred to a ''th-degree'' or ''th-order'' homogeneous function. For example, a homogeneous polynomial of degree defines a homogeneous function of degree . The above definition extends to functions whose domain of a function, domain and codomain are vector spaces over a Field (mathematics), field : a function f : V \to W between two -vector spaces is ''homogeneous'' of degree k if for all nonzero s \in F and v \in V. This definition is often further generalized to f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
State Function
In the thermodynamics of equilibrium, a state function, function of state, or point function for a thermodynamic system is a mathematical function relating several state variables or state quantities (that describe equilibrium states of a system) that depend only on the current equilibrium thermodynamic state of the system (e.g. gas, liquid, solid, crystal, or emulsion), not the path which the system has taken to reach that state. A state function describes equilibrium states of a system, thus also describing the type of system. A state variable is typically a state function so the determination of other state variable values at an equilibrium state also determines the value of the state variable as the state function at that state. The ideal gas law is a good example. In this law, one state variable (e.g., pressure, volume, temperature, or the amount of substance in a gaseous equilibrium system) is a function of other state variables so is regarded as a state function. A stat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intensive And Extensive Properties
Physical or chemical properties of materials and systems can often be categorized as being either intensive or extensive, according to how the property changes when the size (or extent) of the system changes. The terms "intensive and extensive quantities" were introduced into physics by German mathematician Georg Helm in 1898, and by American physicist and chemist Richard C. Tolman in 1917. According to International Union of Pure and Applied Chemistry (IUPAC), an intensive property or intensive quantity is one whose magnitude is independent of the size of the system. An intensive property is not necessarily homogeneously distributed in space; it can vary from place to place in a body of matter and radiation. Examples of intensive properties include temperature, ''T''; refractive index, ''n''; density, ''ρ''; and hardness, ''η''. By contrast, an extensive property or extensive quantity is one whose magnitude is additive for subsystems. Examples include mass, volume and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Constantin Carathéodory
Constantin Carathéodory (; 13 September 1873 – 2 February 1950) was a Greeks, Greek mathematician who spent most of his professional career in Germany. He made significant contributions to real and complex analysis, the calculus of variations, and measure theory. He also created an axiomatic formulation of thermodynamics. Carathéodory is considered one of the greatest mathematicians of his era and the most renowned Greek mathematics, Greek mathematician since Ancient history, antiquity. Origins Constantin Carathéodory was born in 1873 in Berlin to Greeks, Greek parents and grew up in Brussels. His father , a lawyer, served as the Ottoman Empire, Ottoman ambassador to Belgium, St. Petersburg and Berlin. His mother, Despina, née Petrokokkinos, was from the island of Chios. The Carathéodory family, originally from Bosna, Edirne, Bosna, was well established and respected in Istanbul, Constantinople, and its members held many important governmental positions. His grandfather ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Refrigerator
A refrigerator, commonly shortened to fridge, is a commercial and home appliance consisting of a thermal insulation, thermally insulated compartment and a heat pump (mechanical, electronic or chemical) that transfers heat from its inside to its external environment so that its inside is cooled to a temperature below the room temperature. Refrigeration is an essential Food preservation, food storage technique around the world. The low temperature reduces the reproduction rate of bacteria, so the refrigerator lowers the rate of Food spoilage, spoilage. A refrigerator maintains a temperature a few degrees above the freezing point of water. The optimal temperature range for perishable food storage is .Keep your fridge-freezer clean and ice-free ''BBC''. 30 April 2008 A freezer is a specialized refrigerator, or portion of a refrigerator, that maintains its contents’ temperature below the freezing point of water. The refrigerator replaced the icebox, which had been a common househ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Cycle
A thermodynamic cycle consists of linked sequences of thermodynamic processes that involve heat transfer, transfer of heat and work (physics), work into and out of the system, while varying pressure, temperature, and other state variables within the system, and that eventually returns the thermodynamic system, system to its initial state. In the process of passing through a cycle, the working fluid (system) may convert heat from a warm source into useful work, and dispose of the remaining heat to a cold sink, thereby acting as a heat engine. Conversely, the cycle may be reversed and use work to move heat from a cold source and transfer it to a warm sink thereby acting as a heat pump. If at every point in the cycle the system is in thermodynamic equilibrium, the cycle is reversible. Whether carried out reversible or irreversibly, the net entropy change of the system is zero, as entropy is a state function. During a closed cycle, the system returns to its original thermodynamic stat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
László Tisza
László Tisza (July 7, 1907 – April 15, 2009) was a Hungarian-born American physicist who was Professor of Physics Emeritus at MIT. He was a colleague of famed physicists Edward Teller, Lev Landau and Fritz London, and initiated the two-fluid model of liquid helium. United States In 1941, Tisza immigrated to the United States and joined the faculty at the Massachusetts Institute of Technology. His research areas included theoretical physics and the history and philosophy of science, specifically on the foundation of thermodynamics and quantum mechanics. He taught at MIT until 1973. Publications Tisza was the author of the 1966 book, ''Generalized Thermodynamics''. The 1982 publication, ''Physics as Natural Philosophy: Essays in Honor of László Tisza'', was written by Tisza's colleagues and former students in honor of his 75th birthday. Affiliations He was a Fellow of The American Physical Society and American Academy of Arts and Sciences The American Academy o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Max Planck
Max Karl Ernst Ludwig Planck (; ; 23 April 1858 – 4 October 1947) was a German Theoretical physics, theoretical physicist whose discovery of energy quantum, quanta won him the Nobel Prize in Physics in 1918. Planck made many substantial contributions to theoretical physics, but his fame as a physicist rests primarily on his role as the originator of Quantum mechanics, quantum theory and one of the founders of modern physics, which revolutionized understanding of atomic and Subatomic particle, subatomic processes. He is known for the Planck constant, which is of foundational importance for quantum physics, and which he used to derive a set of Unit of measurement, units, today called Planck units, expressed only in terms of fundamental physical constants. Planck was twice president of the German scientific institution Kaiser Wilhelm Society. In 1948, it was renamed the Max Planck Society (Max-Planck-Gesellschaft) and nowadays includes 83 institutions representing a wide range ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
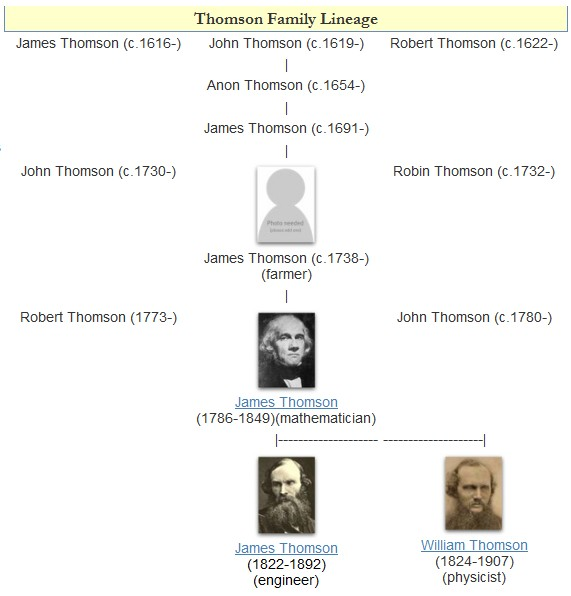
William Thomson, 1st Baron Kelvin
William Thomson, 1st Baron Kelvin (26 June 182417 December 1907), was a British mathematician, Mathematical physics, mathematical physicist and engineer. Born in Belfast, he was the Professor of Natural Philosophy (Glasgow), professor of Natural Philosophy at the University of Glasgow for 53 years, where he undertook significant research on the mathematical analysis of electricity, was instrumental in the formulation of the first and second laws of thermodynamics, and contributed significantly to unifying physics, which was then in its infancy of development as an emerging academic discipline. He received the Royal Society's Copley Medal in 1883 and served as its President of the Royal Society, president from 1890 to 1895. In 1892, he became the first scientist to be elevated to the House of Lords. Absolute temperatures are stated in units of kelvin in Lord Kelvin's honour. While the existence of a coldest possible temperature, absolute zero, was known before his work, Kelvin d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |