|
Thelephoric Acid
Thelephoric acid is a terphenylquinone pigment that is found in several fungi, such as '' Omphalotus subilludens'' and ''Polyozellus multiplex''. Thelephoric acid has been shown to inhibit prolyl endopeptidase, an enzyme that has a role in processing proteins (specifically, amyloid precursor protein) in Alzheimer's disease. Chemicals that inhibit prolyl endopeptidase have attracted research interest due to their potential therapeutic effects. It is derived from atromentin Atromentin is a natural chemical compound found in Agaricomycetes fungi in the orders Agaricales and Thelephorales. It can also be prepared by laboratory synthesis. Chemically, it is a polyphenol and a benzoquinone. Occurrences Atromentin has ..., and its precursor can be from cyclovariegatin. Fragmentation patterns have suggested that polymers of thelephoric acid exists. References {{Reflist, refs= {{Cite journal , vauthors=Hwang JS, Song KS, Kim WG, Lee TH, Koshino H, Yoo ID , title=Polyozellin, a new ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terphenylquinones
Terphenylquinones are fungal dyes from the group of phenyl-substituted ''p''-benzoquinones having the following general structure. General chemical structure of terphenylquinones Also derivatives with a central ''o''-benzoquinone structure are known. Biosynthesis The biosynthesis of terphenylquinones is carried out by dimerization of substituted oxophenylpropanoic acids (phenylpyruvic acids). Occurrence Terphenylquinones are typical constituents of the Boletales. Examples :{, class="wikitable sortable" !Name, , Structure, , CAS-Nr., , Origin , - , Polyporic acid, , , , 548-59-4, , Polypore of the order Aphyllophorales, lichen ''Yarrumia coronata'' , - , Atromentin, , , , 519-67-5, , ''Paxillus atrotomentosus'' (Basidiomycota) , - , Aurantiacin, , , , 548-32-3, , ''Hydnellum aurantiacum'' (Basidiomycota) , - , Phlebiarubron, , , , 7204-23-1, , Cultures of ''Phlebia strigosozonata'' and ''Punctularia atropurpurascens'' (Basidiomycota) , - , Spiromentin B, , , , 121254-56 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclovariegatin
Cyclovariegatin is a pigment. Its chemical name is 1,4-dihydro-2,7,8-trihydroxy-3-(3,4-dihydroxyphenyl)-l,4-dioxodibenzofuran. It is distinguishable by its UV-Vis spectra with maxima at 257, 296, and 430 nm. The variants cyclovariegatin-pentaacetate, cyclovariegatin-2,3',8-triacetate, and cyclovariegatin-2-acetate have also been described. It is derived from atromentin. It has been isolated from the browned skin of ''Suillus grevillei'' var. badius, and becomes the pigment thelephoric acid. See also * Pulvinic acid * Pulvinone * Vulpinic acid Vulpinic acid is a natural product first found in and important in the symbiosis underlying the biology of lichens. It is a simple methyl ester derivative of its parent compound, pulvinic acid, and a close relative of pulvinone, both of which d ... References {{Reflist, refs= Gill, M., and Steglich, W. (1987) Pigments of fungi (Macromycetes). Prog Chem Org Nat Prod 51: 1–317. Elisabeth Jägers, Elisabeth Hillen-Maske, Holger Sch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compounds With 5 Rings
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different chemical element, elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
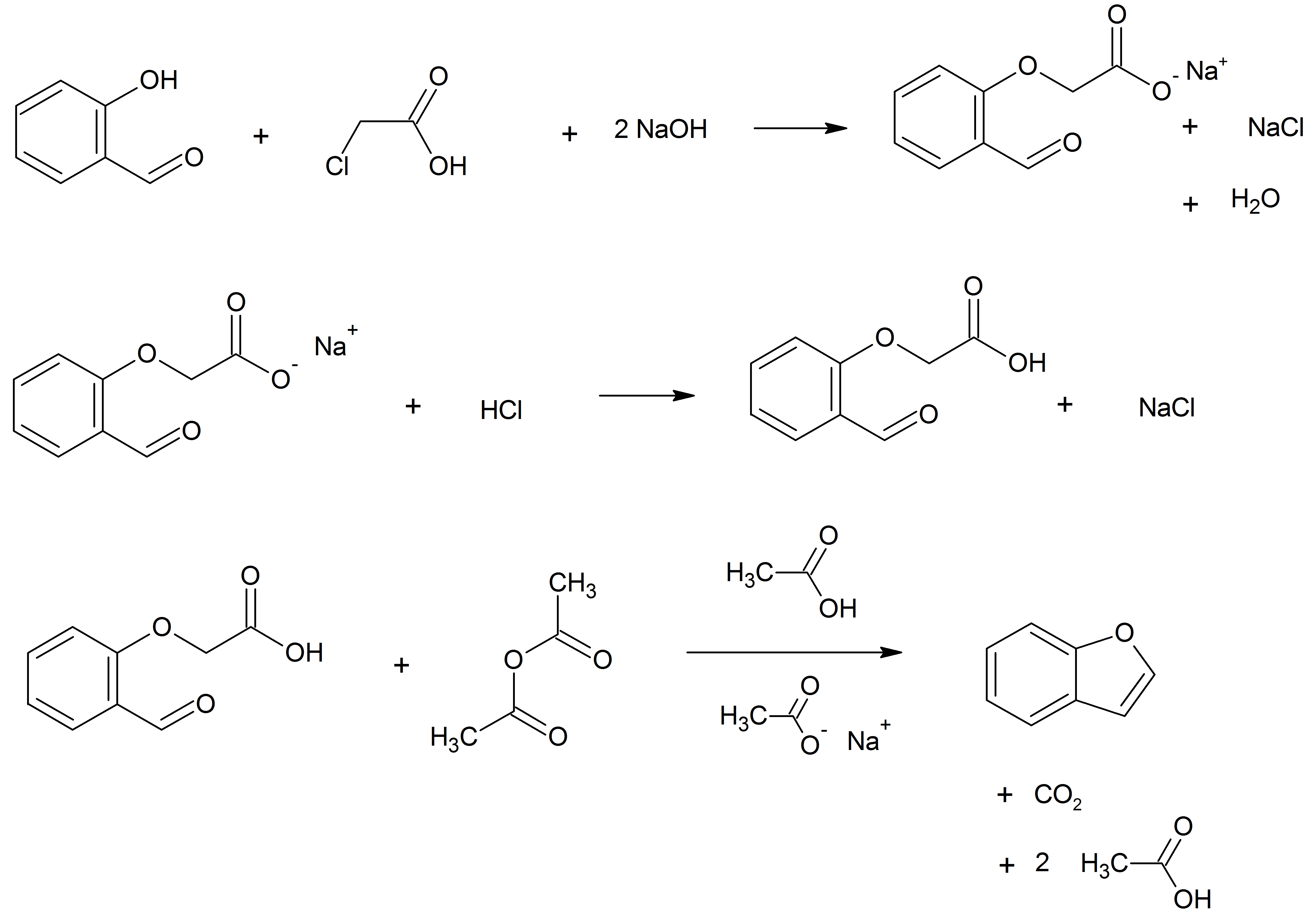
Benzofuran Ethers At The Benzene Ring
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : *Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px *Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px Related compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dibenzofurans
Dibenzofuran is a heterocyclic organic compound with the chemical structure shown at right. It is an aromatic compound that has two benzene rings fused to a central furan ring. All the numbered carbon atoms have a hydrogen atom bonded to each of them. It is a volatile white solid that is soluble in nonpolar organic solvents. It is obtained from coal tar, where it exists as a 1% component.Gerd Collin and Hartmut Höke "Benzofurans" in Ullmann's Encyclopedia of Industrial Chemistry, 2007, Wiley-VCH, Weinheim. Reactions Dibenzofuran is thermally robust with a convenient liquid range. These properties, together with its low toxicity, are exploited by the use of DBF as a heat transfer agent. It undergoes electrophilic reactions, such as halogenation and Friedel-Crafts reactions. Reaction of DBF with butyl lithium results in di lithiation.Ulrich Iserloh, Yoji Oderaotoshi, Shuji Kanemasa, and Dennis P. Curran "Synthesis of (R,R)-4,6-Dibenzofurandiyl-2,2'-Bis (4-Phenyloxazoline) (DBF ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catechols
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amounts. It was first discovered by destructive distillation of the plant extract catechin. About 20,000 tonnes of catechol are now synthetically produced annually as a commodity organic chemical, mainly as a precursor to pesticides, flavors, and fragrances. Catechol occurs as feathery white crystals that are very rapidly soluble in water. Isolation and synthesis Catechol was first isolated in 1839 by Edgar Hugo Emil Reinsch (1809–1884) by distilling it from the solid tannic preparation catechin, which is the residuum of catechu, the boiled or concentrated juice of ''Mimosa catechu'' ('' Acacia catechu''). Upon heating catechin above its decomposition point, a substance that Reinsch first named ''Brenz-Katechusäure'' (burned catechu a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolase Inhibitors
Hydrolase is a class of enzyme that commonly perform as biochemical catalysts that use water to break a chemical bond, which typically results in dividing a larger molecule into smaller molecules. Some common examples of hydrolase enzymes are esterases including lipases, phosphatases, glycosidases, peptidases, and nucleosidases. Esterases cleave ester bonds in lipids and phosphatases cleave phosphate groups off molecules. An example of crucial esterase is acetylcholine esterase, which assists in transforming the neuron impulse into the acetate group after the hydrolase breaks the acetylcholine into choline and acetic acid. Acetic acid is an important metabolite in the body and a critical intermediate for other reactions such as glycolysis. Lipases hydrolyze glycerides. Glycosidases cleave sugar molecules off carbohydrates and peptidases hydrolyze peptide bonds. Nucleosidases hydrolyze the bonds of nucleotides. Hydrolase enzymes are important for the body because they h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinones
The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully Conjugated system, conjugated cyclic diketone, dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone" (thus the name of the class). Other important examples are 1,2-benzoquinone (''ortho''-quinone), 1,4-naphthoquinone and 9,10-anthraquinone. The name is derived from that of quinic acid (with the suffix "-one" indicating a ketone), since it is one of the compounds obtained upon oxidation of quinic acid. Quinic acid, like quinine is obtained from cinchona bark, called quinaquina in the indigenous languages of Peruvian tribes. Properties Quinones are oxidized derivatives of aromatic compounds and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fragmentation (mass Spectrometry)
In mass spectrometry, fragmentation is the dissociation of energetically unstable molecular ions formed from passing the molecules in the ionization chamber of a mass spectrometer. The fragments of a molecule cause a unique pattern in the mass spectrum. These reactions are well documented over the decades and fragmentation pattern is useful to determine the molar weight and structural information of the unknown molecule. Fragmentation that occurs in tandem mass spectrometry experiments has been a recent focus of research, because this data helps facilitate the identification of molecules. Mass spectrometry techniques Fragmentation can occur in the ion source (in-source fragmentation) where it has been used with electron ionization to help identify molecules and, recently (2020), with electrospray ionization it has been shown to provide the same benefit in facilitating molecular identification. Prior to these experiments, electrospray ionization in-source fragmentation was generally ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atromentin
Atromentin is a natural chemical compound found in Agaricomycetes fungi in the orders Agaricales and Thelephorales. It can also be prepared by laboratory synthesis. Chemically, it is a polyphenol and a benzoquinone. Occurrences Atromentin has been found in cultures of '' Clitocybe subilludens'' and in extracts of ''Hydnellum peckii''. The first enzymes in its biosynthesis have been characterized in '' Tapinella panuoides''. One of those is called atromentin synthetase. Biological activities A number of potential biological activities of atromentin have been studied ''in vitro''. Atromentin possesses ''in vitro'' antibacterial activity, inhibiting the enzyme enoyl-acyl carrier protein reductase (essential for the biosynthesis of fatty acids) in the bacteria ''Streptococcus pneumoniae''. Atromentin has been shown to be a smooth muscle stimulant. It also induces apoptosis in isolated human leukemia U937 cells. It is also an anticoagulant Anticoagulants, commonly kno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compounds. Pigments of prehistoric and historic value include ochre, charcoal, and lapis lazuli. Economic impact In 2006, around 7.4 million tons of inorganic, organic, and special pigments were marketed worldwide. Estimated at around US$14.86 billion in 2018 and will rise at over 4.9% CAGR from 2019 to 2026. The global demand for pigments was roughly US$20.5 billion in 2009. According to an April 2018 report by '' Bloomberg Businessweek'', the estimated value of the pigment industry globally is $30 billion. The value of titanium dioxide – used to enhance the white brightness of many products – was placed at $13.2 billion per year, while the color Ferrari red is valued at $300 million each year. Physical princip ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |