|
Tetrathionic Acid
The tetrathionate anion, , is a sulfur oxoanion derived from the compound tetrathionic acid, H2S4O6. Two of the sulfur atoms present in the ion are in oxidation state 0 and two are in oxidation state +5. Alternatively, the compound can be viewed as the adduct resulting from the binding of to SO3. Tetrathionate is one of the polythionates, a family of anions with the formula ''n''(SO3)2sup>2−. Its IUPAC name is ''2-(dithioperoxy)disulfate'', and the name of its corresponding acid is ''2-(dithioperoxy)disulfuric acid''. The Chemical Abstracts Service identifies tetrathionate by the CAS Number 15536-54-6. Formation Tetrathionate is a product of the oxidation of thiosulfate, , by iodine, I2: :2 + I2 → + 2 I− Structure Tetrathionate's structure can be visualized by following three edges of a rectangular cuboid, as in the diagram below. The structure shown is the configuration of in BaS4O6·2H2O and Na2S4O6·2H2O. Dihedral S–S–S–S angles approaching 90° are comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithionite
The dithionite is the oxyanion with the formula 2O4sup>2−. It is commonly encountered as the salt sodium dithionite. For historical reasons, it is sometimes called hydrosulfite, but it contains no hydrogen and is not a sulfite. The dianion has a steric number of 4 and trigonal pyramidal geometry. Production and reactions In its main applications, dithionite is generally prepared in situ by reduction of sulfur dioxide by sodium borohydride, described by the following idealized equation:. : Dithionite is a reducing agent. At pH 7, the potential is −0.66 V vs NHE. Redox occurs with formation of sulfite: : + 2 H2O → 2 + 2 e− + 2 H+ Dithionite undergoes acid hydrolytic disproportionation to thiosulfate and bisulfite: :2 + H2O → + 2 It also undergoes alkaline hydrolytic disproportionation to sulfite and sulfide: :3 Na2S2O4 + 6 NaOH → 5 Na2SO3 + Na2S + 3 H2O It is formally derived from dithionous acid (H2S2O4), but this acid does not exist i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion. In the most common use of the word, this means electrochemical oxidation of metal in reaction with an oxidant such as oxygen, hydrogen or hydroxide. Rusting, the formation of iron oxides, is a well-known example of electrochemical corrosion. This type of damage typically produces oxide(s) or salt(s) of the original metal and results in a distinctive orange colouration. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term "degradation" is more common. Corrosion degrades the useful properties of materials and structures including strength, appearance and permeability to liquids and gases. Many structural ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of the one-electron reduction of dioxygen , which occurs widely in nature. Molecular oxygen (dioxygen) is a diradical containing two unpaired electrons, and superoxide results from the addition of an electron which fills one of the two degenerate molecular orbitals, leaving a charged ionic species with a single unpaired electron and a net negative charge of −1. Both dioxygen and the superoxide anion are free radicals that exhibit paramagnetism. Superoxide was historically also known as "hyperoxide". Salts Superoxide forms salts with alkali metals and alkaline earth metals. The salts caesium superoxide (), rubidium superoxide (), potassium superoxide (), and sodium superoxide () are prepared by the reaction of with the respective alkali me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
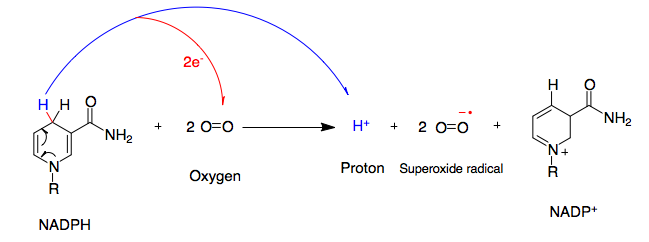
NADPH Oxidase
NADPH oxidase (nicotinamide adenine dinucleotide phosphate oxidase) is a membrane-bound enzyme complex that faces the extracellular space. It can be found in the plasma membrane as well as in the membranes of phagosomes used by neutrophil white blood cells to engulf microorganisms. Human isoforms of the catalytic component of the complex include NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2. Reaction NADPH oxidase catalyzes the production of a superoxide free radical by transferring one electron to oxygen from NADPH. : Types In mammals, NADPH oxidase is found in two types: one in white blood cells (neutrophilic) and the other in vascular cells, differing in biochemical structure and functions. Neutrophilic NADPH oxidase produces superoxide almost instantaneously, whereas the vascular enzyme produces superoxide in minutes to hours. Moreover, in white blood cells, superoxide has been found to transfer electrons across the membrane to extracellular oxygen, while in vascula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
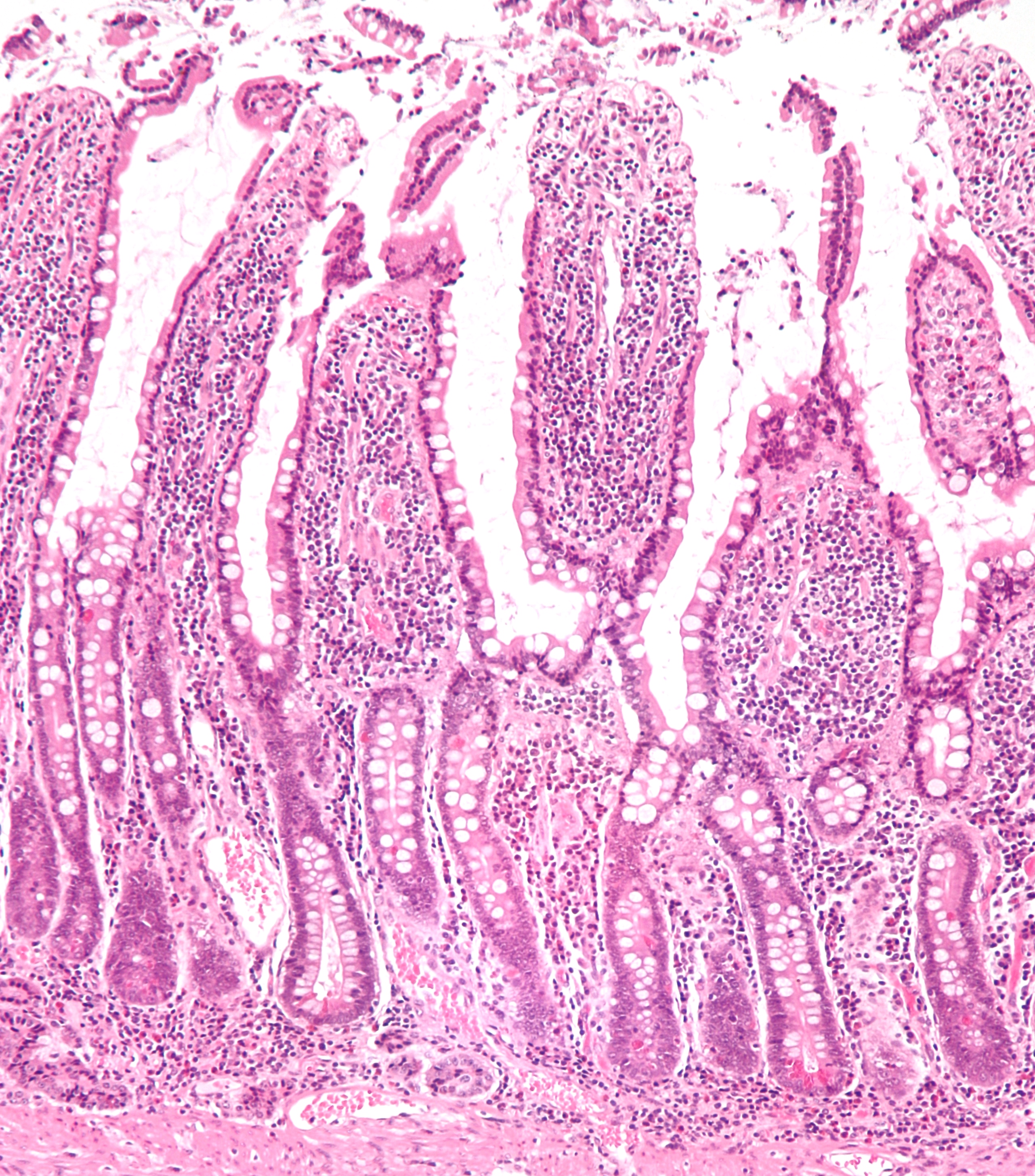
Small Intestine
The small intestine or small bowel is an organ in the gastrointestinal tract where most of the absorption of nutrients from food takes place. It lies between the stomach and large intestine, and receives bile and pancreatic juice through the pancreatic duct to aid in digestion. The small intestine is about long and folds many times to fit in the abdomen. Although it is longer than the large intestine, it is called the small intestine because it is narrower in diameter. The small intestine has three distinct regions – the duodenum, jejunum, and ileum. The duodenum, the shortest, is where preparation for absorption through small finger-like protrusions called villi begins. The jejunum is specialized for the absorption through its lining by enterocytes: small nutrient particles which have been previously digested by enzymes in the duodenum. The main function of the ileum is to absorb vitamin B12, bile salts, and whatever products of digestion that were not absorbed by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salmonella Enterica
''Salmonella enterica'' (formerly ''Salmonella choleraesuis'') is a rod-headed, flagellate, facultative anaerobic, Gram-negative bacterium and a species of the genus ''Salmonella''. A number of its serovars are serious human pathogens. Epidemiology Most cases of salmonellosis are caused by food infected with ''S. enterica'', which often infects cattle and poultry, though other animals such as domestic cats and hamsters have also been shown to be sources of infection in humans. Investigations of vacuum cleaner bags have shown that households can act as a reservoir of the bacterium; this is more likely if the household has contact with an infection source (i.e., members working with cattle or in a veterinary clinic). Raw chicken eggs and goose eggs can harbor ''S. enterica'', initially in the egg whites, although most eggs are not infected. As the egg ages at room temperature, the yolk membrane begins to break down and ''S. enterica'' can spread into the yolk. Refrigeration a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stainless Steel
Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's corrosion resistance, resistance to corrosion results from the chromium, which forms a Passivation (chemistry), passive film that can protect the material and self-healing material, self-heal in the presence of oxygen. The alloy's properties, such as luster and resistance to corrosion, are useful in many applications. Stainless steel can be rolled into Sheet metal, sheets, plates, bars, wire, and tubing. These can be used in cookware, cutlery, surgical instruments, major appliances, vehicles, construction material in large buildings, industrial equipment (e.g., in paper mills, chemical plants, water treatment), and storage tanks and tankers for chemicals and food products. The biological cleanability of stainless steel is superior to both alumi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
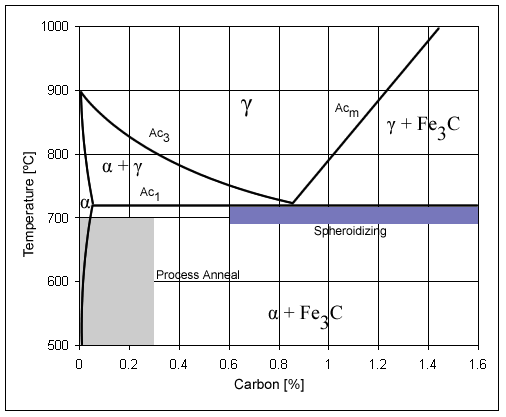
Carbon Steel
Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states: * no minimum content is specified or required for chromium, cobalt, molybdenum, nickel, niobium, titanium, tungsten, vanadium, zirconium, or any other element to be added to obtain a desired alloying effect; * the specified minimum for copper does not exceed 0.40%; * or the maximum content specified for any of the following elements does not exceed the percentages noted: manganese 1.65%; silicon 0.60%; copper 0.60%. The term ''carbon steel'' may also be used in reference to steel which is not stainless steel; in this use carbon steel may include alloy steels. High carbon steel has many different uses such as milling machines, cutting tools (such as chisels) and high strength wires. These applications require a much finer microstructure, which improves the toughness. Carbon steel is a popular metal choic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pitting Corrosion
Pitting corrosion, or pitting, is a form of extremely localized corrosion that leads to the random creation of small holes in metal. The driving power for pitting corrosion is the depassivation of a small area, which becomes anodic (oxidation reaction) while an unknown but potentially vast area becomes cathodic (reduction reaction), leading to very localized galvanic corrosion. The corrosion penetrates the mass of the metal, with a limited diffusion of ions. Another term arises, pitting factor, which is defined as the ratio of the depth of the deepest pit (resulting due to corrosion) to the average penetration, which can be calculated based on the weight loss. Development and kinetics of pitting According to Frankel (1998) who performed a review on pitting corrosion, it develops in three successive steps: (or nucleation) by breakdown of the passive film protecting the metal surface from oxidation, (2) growth of metastable pits (growing up to the micron scale and then repassiva ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood. Chemical nature Inorganic chemistry Hydrates are inorganic salts "containing water molecules combined in a definite ratio as an integral part of the crystal" that are either bound to a metal center or that have crystallized with the metal complex. Such hydrates are also said to contain ''water of crystallization'' or ''water of hydration''. If the water is heavy water in which the constituent hydrogen is the isotope deuterium, then the term ''deuterate'' may be used in place of ''hydrate''. A colorful example is cobalt(II) chloride, which turns from blue to red upon hydration, and can therefore be used as a water indicator. The notation "''hydrated compound''⋅''n''", where ''n'' is the number of water molecules per formula un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


_chloride.jpg)