|
Tenebrescence
Photochromism is the reversible transformation of a chemical species (photoswitch) between two forms by the absorption of electromagnetic radiation (photoisomerization), where the two forms have different absorption spectra. In plain language, this can be described as a reversible change of color upon exposure to light. Applications Sunglasses One of the most famous reversible photochromic applications is color changing lenses for sunglasses. The largest limitation in using photochromic technology is that the materials cannot be made stable enough to withstand thousands of hours of outdoor exposure so long-term outdoor applications are not appropriate at this time. The switching speed of photochromic dyes is highly sensitive to the rigidity of the environment around the dye. As a result, they switch most rapidly in solution and slowest in the rigid environment like a polymer lens. In 2005 it was reported that attaching flexible polymers with low glass transition temperature (f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Copenhagen Department Of Chemistry
The Department of Chemistry ( da, Kemisk Institut) is a department under the Faculty of Science, University of Copenhagen. It is the largest basic research institute in chemistry in Denmark, and is responsible for the teaching of chemistry at all levels at the University of Copenhagen's Faculty of Science: from undergraduate courses in chemistry to Ph.D.-level courses. Its office is located at the University of Copenhagen's North Campus. History Research and education in chemistry has been conducted at the department since 1778, when the first Laboratorium Chymicum was established. Since then, the chemists have moved several times. In 1962, Universitetets Kemiske Laboratorium moved to its present location in the H.C. Ørsted Institute (HCØ), named after Hans Christian Ørsted who discovered electromagnetism In physics, electromagnetism is an interaction that occurs between particles with electric charge. It is the second-strongest of the four fundamental interaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociation (chemistry)
Dissociation in chemistry is a general process in which molecules (or ionic compounds such as salts, or complexes) separate or split into other things such as atoms, ions, or radicals, usually in a reversible manner. For instance, when an acid dissolves in water, a covalent bond between an electronegative atom and a hydrogen atom is broken by heterolytic fission, which gives a proton (H+) and a negative ion. Dissociation is the opposite of association or recombination. Dissociation constant For reversible dissociations in a chemical equilibrium :AB A + B the dissociation constant ''K''d is the ratio of dissociated to undissociated compound :K_d = \mathrm where the brackets denote the equilibrium concentrations of the species. Dissociation degree The dissociation degree \alpha is the fraction of original solute molecules that have dissociated. It is usually indicated by the Greek symbol α. More accurately, degree of dissociation refers to the amount of solute dissoci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Transfer
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 y ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pericyclic Reaction
In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted fashion, and the bond orbitals involved in the reaction overlap in a continuous cycle at the transition state. Pericyclic reactions stand in contrast to ''linear reactions'', encompassing most organic transformations and proceeding through an acyclic transition state, on the one hand and '' coarctate reactions'', which proceed through a doubly cyclic, concerted transition state on the other hand. Pericyclic reactions are usually rearrangement or addition reactions. The major classes of pericyclic reactions are given in the table below (the three most important classes are shown in bold). Ene reactions and cheletropic reactions are often classed as group transfer reactions and cycloadditions/cycloeliminations, respectively, while dyotropic reactions and group transfer reactions (if ene reactions are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stilbene
Stilbene may refer to one of the two stereoisomers of 1,2-diphenylethene: * (''E'')-Stilbene (''trans'' isomer) * (''Z'')-Stilbene (''cis'' isomer) See also * Stilbenoid Stilbenoids are hydroxylated derivatives of stilbene. They have a C6–C2–C6 structure. In biochemical terms, they belong to the family of phenylpropanoids and share most of their biosynthesis pathway with chalcones. Most stilbenoids are prod ...s, a class of molecules found in plants * 1,1-Diphenylethylene {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
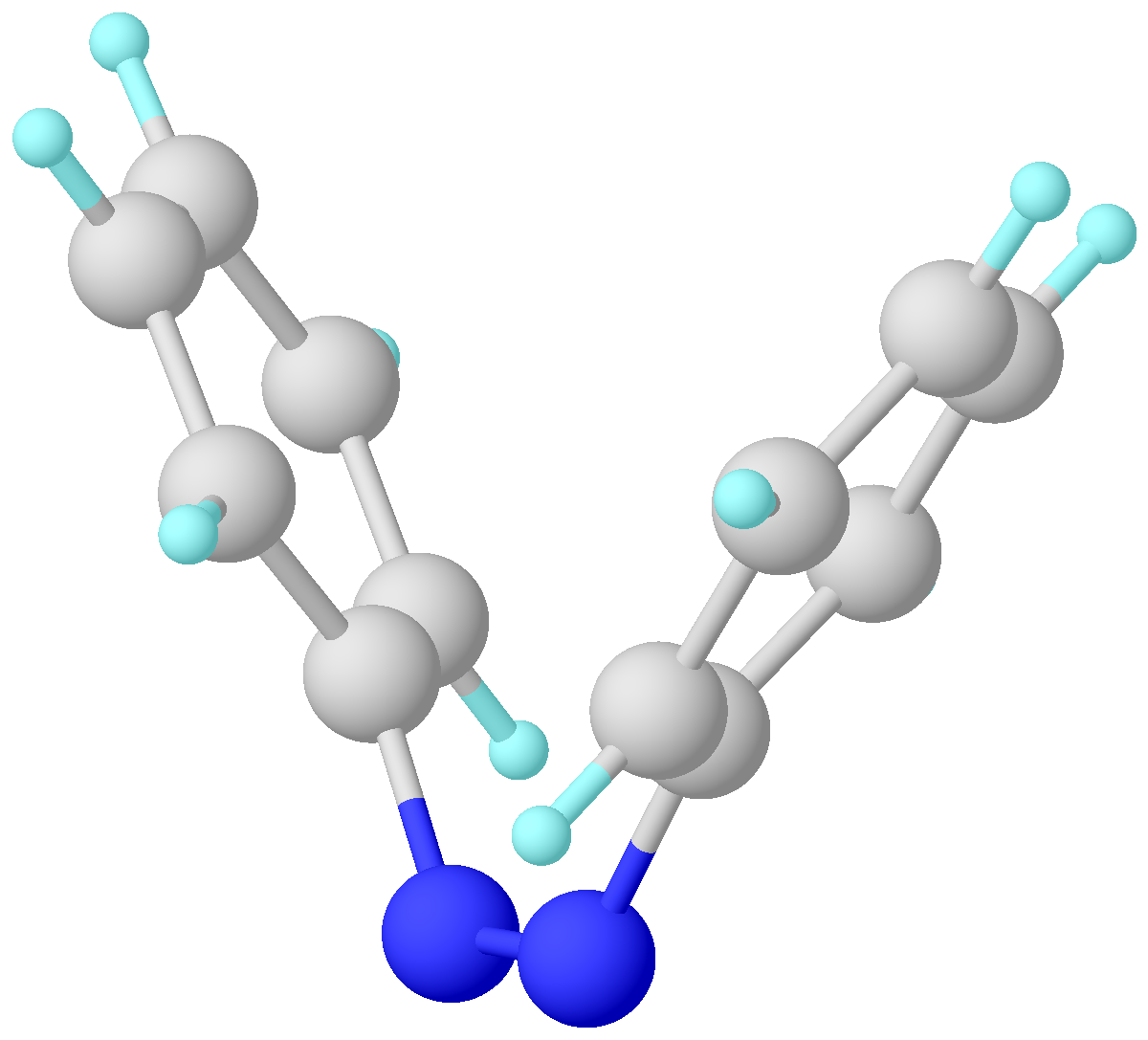
Azobenzene
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes. Structure and synthesis ''trans''-Azobenzene is planar. The N-N distance is 1.189 Å. ''cis''-Azobenzene is nonplanar with a C-N=N-C dihedral angle of 173.5°. The N-N distance is 1.251 Å. Azobenzene was first described by Eilhard Mitscherlich in 1834. Yellowish-red crystalline flakes of azobenzene were obtained in 1856. Its original preparation is similar to the modern one. According to the 1856 method, nitrobenzene is reduced by iron filings in the presence of acetic acid. In the modern synthesis, zinc is the reductant in the presence of a base. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the isomerization occurs intramolecularly it may be called a rearrangement reaction. When the activation energy for the isomerization reaction is sufficiently small, both isomers will exist in a temperature-dependent equilibrium with each other. Many values of the standard free energy difference, \Delta G^\circ, have been calculated, with good agreement between observed and calculated data. Examples and applications Alkanes Skeletal isomerization occurs in the cracking process, used in the petrochemical industry. As well as reducing the average chain length, straight-chain hydrocarbons are converted to branched isomers in the process, as illustrated the following reaction of ''n''-butane to ''i''-butane. :\overset -> ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electromagnetic Spectrum
The electromagnetic spectrum is the range of frequencies (the spectrum) of electromagnetic radiation and their respective wavelengths and photon energies. The electromagnetic spectrum covers electromagnetic waves with frequencies ranging from below one hertz to above 1025 hertz, corresponding to wavelengths from thousands of kilometers down to a fraction of the size of an atomic nucleus. This frequency range is divided into separate bands, and the electromagnetic waves within each frequency band are called by different names; beginning at the low frequency (long wavelength) end of the spectrum these are: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays at the high-frequency (short wavelength) end. The electromagnetic waves in each of these bands have different characteristics, such as how they are produced, how they interact with matter, and their practical applications. There is no known limit for long and short wavelengths. Extreme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorption Band
According to quantum mechanics, atoms and molecules can only hold certain defined quantities of energy, or exist in specific states. When such quanta of electromagnetic radiation are emitted or absorbed by an atom or molecule, energy of the radiation changes the state of the atom or molecule from an initial state to a final state. An absorption band is a range of wavelengths, frequencies or energies in the electromagnetic spectrum which are characteristic of a particular transition from initial to final state in a substance. Overview According to quantum mechanics, atoms and molecules can only hold certain defined quantities of energy, or exist in specific states. When electromagnetic radiation is absorbed by an atom or molecule, the energy of the radiation changes the state of the atom or molecule from an initial state to a final state. The number of states in a specific energy range is discrete for gaseous or diluted systems, with discrete energy levels. Condensed s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photochemical Reaction
Organic photochemistry encompasses organic reactions that are induced by the action of light. The absorption of ultraviolet light by organic molecules often leads to reactions. In the earliest days, sunlight was employed, while in more modern times ultraviolet lamps are employed. Organic photochemistry has proven to be a very useful synthetic tool. Complex organic products can be obtained simply. History Early examples were often uncovered by the observation of precipitates or color changes from samples that were exposed to sunlights. The first reported case was by Ciamician that sunlight converted santonin to a yellow photoproduct: An early example of a precipitate was the photodimerization of anthracene, characterized by Yulii Fedorovich Fritzsche and confirmed by Elbs. Similar observations focused on the dimerization of cinnamic acid to truxillic acid. Many photodimers are now recognized, e.g. pyrimidine dimer, thiophosgene, diamantane. Another example was uncovered by Egb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Retinal
Retinal (also known as retinaldehyde) is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision). Some microorganisms use retinal to convert light into metabolic energy. In fact, a recent study suggests most living organisms on our planet ~3 billion years ago used retinal to convert sunlight into energy rather than chlorophyll. Since retinal absorbs mostly green light and transmits purple light, this gave rise to the Purple Earth Hypothesis. There are many forms of vitamin A — all of which are converted to retinal, which cannot be made without them. Retinal itself is considered to be a form of vitamin A when eaten by an animal. The number of different molecules that can be converted to retinal varies from species to species. Retinal was originally called retinene, and was renamed after it was discovered to be vitamin A aldehyde. Vertebrate animals ingest r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |