|
Talipexole
Talipexole (B-HT920, Domnin) is a dopamine agonist that is marketed as a treatment for Parkinson's Disease in Japan by Boehringer Ingelheim; it was introduced in 1996. As of December 2014 it was not approved for marketing in the US nor in Europe. Talipexole is a D2 dopamine receptor agonist and interacts both pre- and post-synaptic receptors. It also is an α2-adrenergic agonist. The main side effects are drowsiness, dizziness, hallucinations and minor gastrointestinal complaints. In 2008 the Japanese Ministry of Health, Labour, and Welfare mandated that Boehringer add a warning to the label concerning the risk of sudden onset of sleep.Japanese Ministry of Health, Labour and Welfare March 200Pharmaceuticals and Medical Devices Safety Information No. 245/ref> Synthesis The N-alkylation of azepan-4-one 05416-56-6(1) with allyl bromide in the presence of potassium carbonate gives 1-allyl-azepan-4-one (2). This is halogenated with molecular bromine in acetic acid to give ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Talipexole Synthesis
Talipexole (B-HT920, Domnin) is a dopamine agonist that is marketed as a treatment for Parkinson's Disease in Japan by Boehringer Ingelheim; it was introduced in 1996. As of December 2014 it was not approved for marketing in the US nor in Europe. Talipexole is a D2 dopamine receptor agonist and interacts both pre- and post-synaptic receptors. It also is an α2-adrenergic agonist. The main side effects are drowsiness, dizziness, hallucinations and minor gastrointestinal complaints. In 2008 the Japanese Ministry of Health, Labour, and Welfare mandated that Boehringer add a warning to the label concerning the risk of sudden onset of sleep.Japanese Ministry of Health, Labour and Welfare March 200Pharmaceuticals and Medical Devices Safety Information No. 245/ref> Synthesis The N-alkylation of azepan-4-one 05416-56-6(1) with allyl bromide in the presence of potassium carbonate gives 1-allyl-azepan-4-one (2). This is halogenated with molecular bromine in acetic acid to give 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
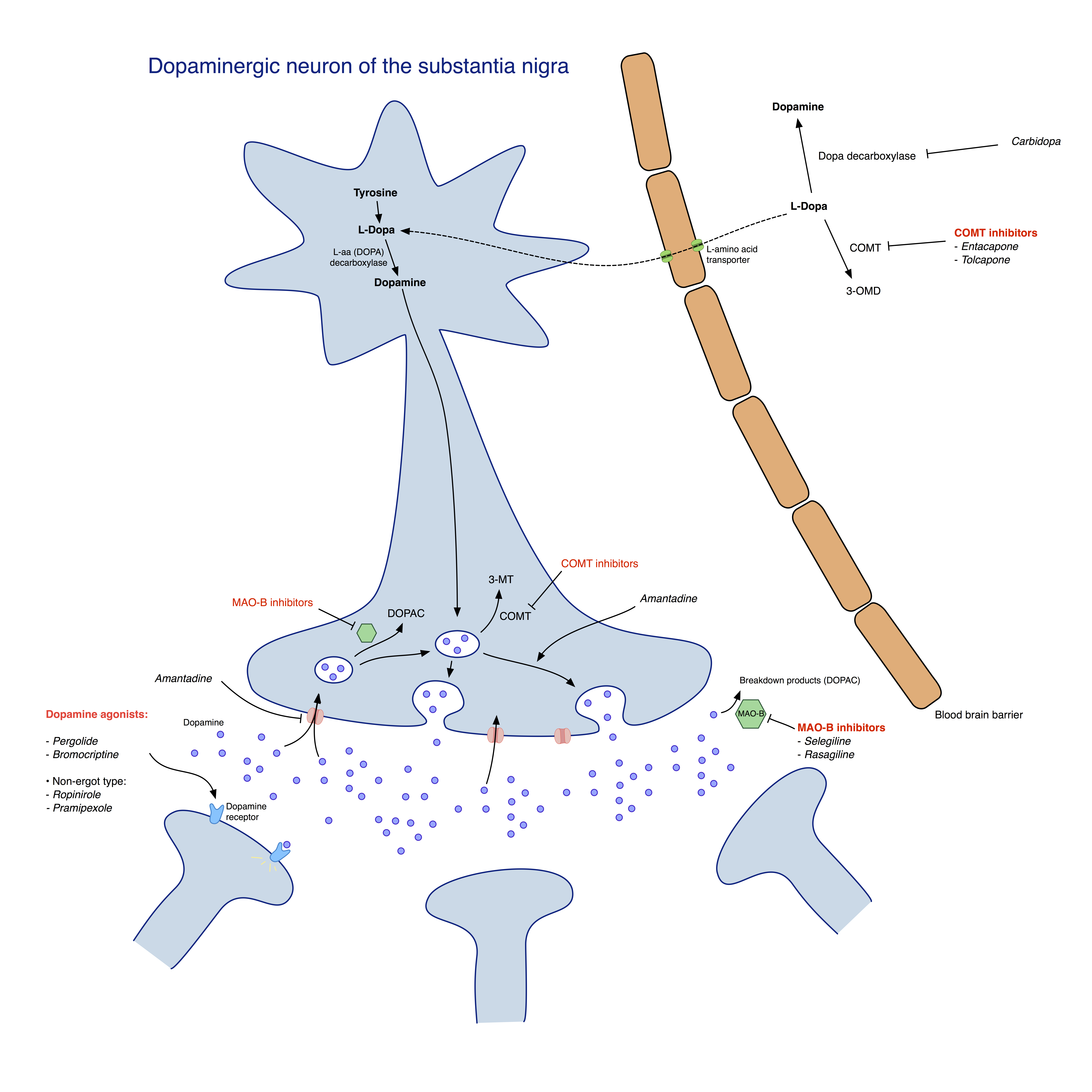
Dopamine Agonist
A dopamine agonist (DA) is a compound that activates dopamine receptors. There are two families of dopamine receptors, D2-like and D1-like, and they are all G protein-coupled receptors. D1- and D5-receptors belong to the D1-like family and the D2-like family includes D2, D3 and D4 receptors. Dopamine agonists are primarily used to treat Parkinson's disease. They are also used, to a far lesser extent, in treating hyperprolactinemia and restless legs syndrome. They are not intended for treatment of clinical depression, and studies have shown severe detrimental side effects resulting from off-label use of dopamine agonists in treating depression, particularly in their tendency to produce impulse control disorders and extreme cases of withdrawal syndrome. Medical uses Parkinson's disease Dopamine agonists are mainly used in the treatment of Parkinson's disease. The cause of Parkinson's is not fully known but genetic factors, for example specific genetic mutations, and environmen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Management Of Parkinson's Disease
In the management of Parkinson's disease, due to the chronic nature of Parkinson's disease (PD), a broad-based program is needed that includes patient and family education, support-group services, general wellness maintenance, exercise, and nutrition. At present, no cure for the disease is known, but medications or surgery can provide relief from the symptoms. While many medications treat Parkinson's, none actually reverses the effects of the disease. Furthermore, the gold-standard treatment varies with the disease state. People with Parkinson's, therefore, often must take a variety of medications to manage the disease's symptoms. Several medications currently in development seek to better address motor fluctuations and nonmotor symptoms of PD. However, none is yet on the market with specific approval to treat Parkinson's. Medication The main families of drugs useful for treating motor symptoms are levodopa, dopamine agonists, and MAO-B inhibitors. The most commonly used treatm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boehringer Ingelheim
C.H. Boehringer Sohn AG & Co. is the parent company of the Boehringer Ingelheim group, which was founded in 1885 by Albert Boehringer in Ingelheim am Rhein, Germany. As of 2018, Boehringer Ingelheim is one of the world's largest pharmaceutical companies, and the largest private one. Headquartered in Ingelheim, it operates globally with 146 affiliates and more than 47,700 employees. Unlike most large pharmaceutical companies which are listed, the company is private and fully owned by the Boehringer, Liebrecht and von Baumbach families. The company's key areas of interest are: respiratory diseases, metabolism, immunology, oncology and diseases of the central nervous system. Boehringer Ingelheim is a full member of the European Federation of Pharmaceutical Industries and Associations (EFPIA). The corporate logo of Boehringer Ingelheim depicts a stylized rendition of the central section of the imperial palace of Charlemagne. History 1885–1999 *1885: Albert Boehringer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine Receptor Agonist
A dopamine agonist (DA) is a compound that activates dopamine receptors. There are two families of dopamine receptors, D2-like and D1-like, and they are all G protein-coupled receptors. D1- and D5-receptors belong to the D1-like family and the D2-like family includes D2, D3 and D4 receptors. Dopamine agonists are primarily used to treat Parkinson's disease. They are also used, to a far lesser extent, in treating hyperprolactinemia and restless legs syndrome. They are not intended for treatment of clinical depression, and studies have shown severe detrimental side effects resulting from off-label use of dopamine agonists in treating depression, particularly in their tendency to produce impulse control disorders and extreme cases of withdrawal syndrome. Medical uses Parkinson's disease Dopamine agonists are mainly used in the treatment of Parkinson's disease. The cause of Parkinson's is not fully known but genetic factors, for example specific genetic mutations, and environment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-adrenergic Agonist
The adrenergic receptors or adrenoceptors are a class of G protein-coupled receptors that are targets of many catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) produced by the body, but also many medications like beta blockers, beta-2 (β2) agonists and alpha-2 (α2) agonists, which are used to treat high blood pressure and asthma, for example. Many cells have these receptors, and the binding of a catecholamine to the receptor will generally stimulate the sympathetic nervous system (SNS). The SNS is responsible for the fight-or-flight response, which is triggered by experiences such as exercise or fear-causing situations. This response dilates pupils, increases heart rate, mobilizes energy, and diverts blood flow from non-essential organs to skeletal muscle. These effects together tend to increase physical performance momentarily. History By the turn of the 19th century, it was agreed that the stimulation of sympathetic nerves could cause diff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl Bromide
Allyl bromide (3-bromopropene) is an organic halide. It is an alkylating agent used in synthesis of polymers, pharmaceuticals, synthetic perfumes and other organic compounds. Physically, allyl bromide is a colorless liquid with an irritating and persistent smell, however, commercial samples are yellow or brown. Allyl bromide is more reactive but more expensive than allyl chloride, and these considerations guide its use. Preparation Allyl bromide is produced commercially from allyl alcohol and hydrobromic acid: :CH2=CHCH2OH + HBr → CH2=CHCH2Br + H2O It can also be prepared by the halogen-exchange reaction between allyl chloride and hydrobromic acid or by the allylic bromination of propene. Reactions and uses Electrophilic properties Allyl bromide is an electrophilic alkylating agent. It reacts with nucleophiles, such as amines, carbanions, alkoxides, etc., to introduce the allyl group: :CH2=CHCH2Br + Nu− → CH2=CHCH2Nu + Br− (Nu− is a nucleophile) It is used in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiourea
Thiourea () is an organosulfur compound with the formula and the structure . It is structurally similar to urea (), except that the oxygen atom is replaced by a sulfur atom (as implied by the ''thio-'' prefix); however, the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. "Thioureas" refer to a broad class of compounds with the general structure . Thioureas are related to thioamides, e.g. , where R is methyl, ethyl, etc. Structure and bonding Thiourea is a planar molecule. The C=S bond distance is 1.71 Å. The C-N distances average 1.33 Å. The weakening of the C-S bond by C-N pi-bonding is indicated by the short C=S bond in thiobenzophenone, which is 1.63 Å. Thiourea occurs in two tautomeric forms, of which the thione form predominates in aqueous solutions. The equilibrium constant has been calculated as ''K''eq is . The thiol form, which is also known as an isothiourea, can be encountered in substituted compounds such as i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pramipexole
Pramipexole, sold under the brand Mirapex among others, is medication used to treat Parkinson's disease (PD) and restless legs syndrome (RLS). In Parkinson's disease it may be used alone or together with levodopa. It is taken by mouth. Pramipexole is a dopamine agonist of the non-ergoline class. Studies have shown detrimental side effects resulting from off-label use of pramipexole or other dopamine agonists in treating clinical depression. Pramipexole was approved for medical use in the United States in 1997. Use in pregnancy and breastfeeding is of unclear safety. It is available as a generic medication. In 2020, it was the 193rd most commonly prescribed medication in the United States, with more than 2million prescriptions. Medical uses Pramipexole is used in the treatment of Parkinson's disease (PD) and restless legs syndrome (RLS). Use in pregnancy and breastfeeding is of unclear safety. It is occasionally prescribed off-label for depression. Its effectiveness as an an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-1 Blockers
Alpha 1 or Alpha-1 may refer to: *Alpha-1 adrenergic receptor, a G protein-coupled receptor *Alpha-1 antitrypsin, a protein **Alpha-1 antitrypsin deficiency, a genetic disorder *Alpha-1-fetoprotein or Alpha-fetoprotein, a protein *Alpha-One, a fictional spacecraft in '' Buzz Lightyear of Star Command: The Adventure Begins'' * ''Alpha 1'' (Robert Silverberg anthology), a 1970 book See also * * *Alpha (other) *AMY1A Alpha-amylase 1 is an enzyme that in humans is encoded by the ''AMY1A'' gene In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth' ... or Alpha-1A or, an enzyme found in humans and other mammals * List of A1 genes, proteins or receptors {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
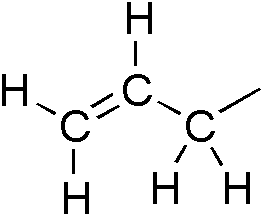
Allyl Compounds
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |