|
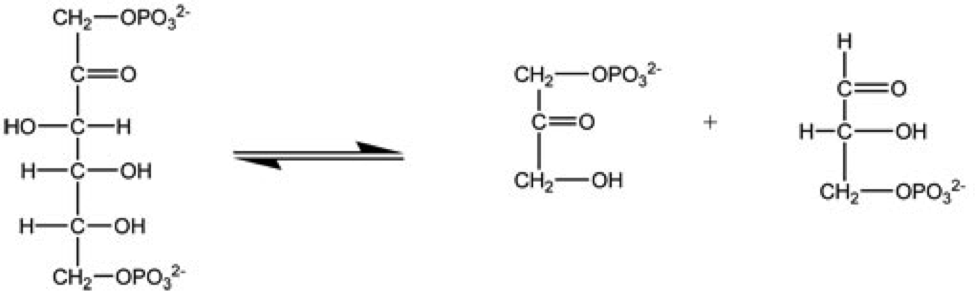
TRNA Splicing Endonuclease
tRNA-intron lyase (EC 4.6.1.16, tRNA intron endonuclease, transfer ribonucleate intron endoribonuclease, tRNA splicing endonuclease, splicing endonuclease, tRNATRPintron endonuclease, transfer splicing endonuclease; systematic name pretRNA lyase (intron-removing; cyclic-2′,3′-phosphate-forming)) is an enzyme. As an endonuclease enzyme, tRNA-intron lyase is responsible for RNA splicing, splicing phosphodiester bonds within Non-coding RNA, non-coding ribonucleic acid chains. These non-coding RNA molecules form Transfer RNA, tRNA molecules after being processed, and this is dependent on tRNA-intron lyase to Spliceosome, splice the pretRNA. tRNA processing is an important post-transcriptional modification necessary for tRNA maturation because it locates and removes introns in the pretRNA. This enzyme Catalysis, catalyses the following Chemical reaction, chemical reaction: : pretRNA = a 3′-half-tRNA molecule with a 5′-OH end + a 5′-half-tRNA molecule with a 2′,3′-cyclic ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Subunit
In structural biology, a protein subunit is a polypeptide chain or single protein molecule that assembles (or "''coassembles''") with others to form a protein complex. Large assemblies of proteins such as viruses often use a small number of types of protein subunits as building blocks. A subunit is often named with a Greek or Roman letter, and the numbers of this type of subunit in a protein is indicated by a subscript. For example, ATP synthase has a type of subunit called α. Three of these are present in the ATP synthase molecule, leading to the designation α3. Larger groups of subunits can also be specified, like α3β3-hexamer and c-ring. Naturally-occurring proteins that have a relatively small number of subunits are referred to as oligomeric.Quote: ''Oligomer molecule: A molecule of intermediate relative molecular mass, the structure of which essentially comprises a small plurality of units derived, actually or conceptually, from molecules of lower relative molecular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microcephaly
Microcephaly (from New Latin ''microcephalia'', from Ancient Greek μικρός ''mikrós'' "small" and κεφαλή ''kephalé'' "head") is a medical condition involving a smaller-than-normal head. Microcephaly may be present at birth or it may develop in the first few years of life. Since brain growth is correlated with head growth, people with this disorder often have an intellectual disability, poor motor function, poor speech, abnormal facial features, seizures and dwarfism. The disorder is caused by a disruption to the genetic processes that form the brain early in pregnancy, though the cause is not identified in most cases. Many genetic syndromes can result in microcephaly, including chromosomal and single-gene conditions, though almost always in combination with other symptoms. Mutations that result solely in microcephaly (primary microcephaly) exist but are less common. External toxins to the embryo, such as alcohol during pregnancy or vertically transmitted infec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
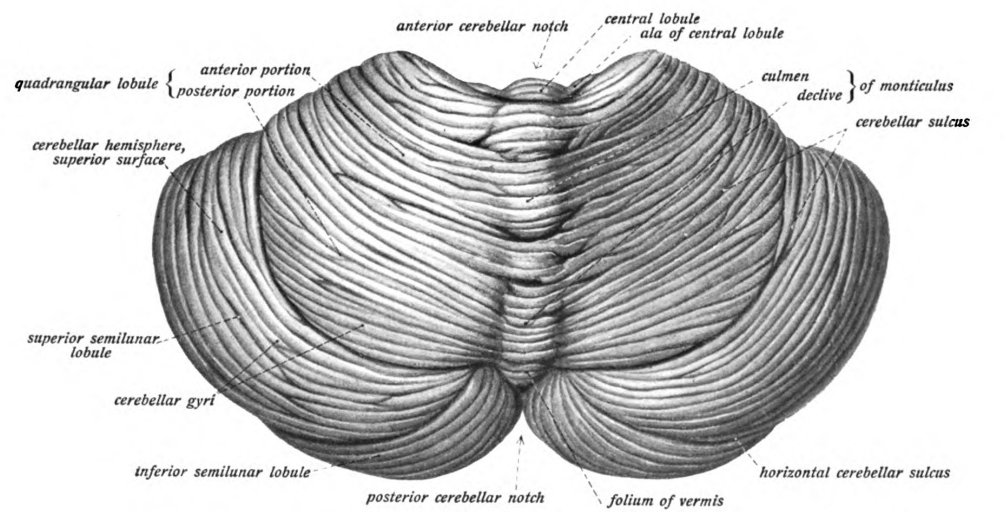
Pons
The pons (from Latin , "bridge") is part of the brainstem that in humans and other bipeds lies inferior to the midbrain, superior to the medulla oblongata and anterior to the cerebellum. The pons is also called the pons Varolii ("bridge of Varolius"), after the Italian anatomist and surgeon Costanzo Varolio (1543–75). This region of the brainstem includes neural pathways and tracts that conduct signals from the brain down to the cerebellum and medulla, and tracts that carry the sensory signals up into the thalamus.Saladin Kenneth S.(2007) Anatomy & physiology the unity of form and function. Dubuque, IA: McGraw-Hill Structure The pons is in the brainstem situated between the midbrain and the medulla oblongata, and in front of the cerebellum. A separating groove between the pons and the medulla is the inferior pontine sulcus. The superior pontine sulcus separates the pons from the midbrain. The pons can be broadly divided into two parts: the basilar part of the pons (ventral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerebellum
The cerebellum (Latin for "little brain") is a major feature of the hindbrain of all vertebrates. Although usually smaller than the cerebrum, in some animals such as the mormyrid fishes it may be as large as or even larger. In humans, the cerebellum plays an important role in motor control. It may also be involved in some cognition, cognitive functions such as attention and language as well as emotion, emotional control such as regulating fear and pleasure responses, but its movement-related functions are the most solidly established. The human cerebellum does not initiate movement, but contributes to Motor coordination, coordination, precision, and accurate timing: it receives input from sensory systems of the spinal cord and from other parts of the brain, and integrates these inputs to fine-tune motor activity. Cerebellar damage produces disorders in Fine motor skill, fine movement, Equilibrioception, equilibrium, Human positions, posture, and motor learning in humans. Anatomica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atrophy
Atrophy is the partial or complete wasting away of a part of the body. Causes of atrophy include mutations (which can destroy the gene to build up the organ), poor nourishment, poor circulation, loss of hormonal support, loss of nerve supply to the target organ, excessive amount of apoptosis of cells, and disuse or lack of exercise or disease intrinsic to the tissue itself. In medical practice, hormonal and nerve inputs that maintain an organ or body part are said to have ''trophic'' effects. A diminished muscular trophic condition is designated as ''atrophy''. Atrophy is reduction in size of cell, organ or tissue, after attaining its normal mature growth. In contrast, hypoplasia is the reduction in the cellular numbers of an organ, or tissue that has not attained normal maturity. Atrophy is the general physiological process of reabsorption and breakdown of tissues, involving apoptosis. When it occurs as a result of disease or loss of trophic support because of other diseases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pontocerebellar Hypoplasia
Pontocerebellar hypoplasia (PCH) is a heterogeneous group of rare neurodegenerative disorders caused by genetic mutations and characterised by progressive atrophy of various parts of the brain such as the cerebellum or brainstem (particularly the pons). Where known, these disorders are inherited in an autosomal recessive fashion. There is no known cure for PCH. Signs and symptoms There are different signs and symptoms for different forms of pontocerebellar hypoplasia, at least six of which have been described by researchers. All forms involve abnormal development of the brain, leading to slow development, movement problems, and intellectual impairment. The following values seem to be aberrant in children with CASK gene defects: lactate, pyruvate, 2-ketoglutaric acid, adipic acid, and suberic acid which seems to support the thesis that CASK affects mitochondrial function. Causes Pontocerebellar hypoplasia is caused by mutations in genes including VRK1 (PCH1); TSEN2, TSEN3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Splice Site
RNA splicing is a process in molecular biology where a newly-made precursor messenger RNA (pre-mRNA) transcript is transformed into a mature messenger RNA (mRNA). It works by removing all the introns (non-coding regions of RNA) and ''splicing'' back together exons (coding regions). For nuclear-encoded genes, splicing occurs in the nucleus either during or immediately after transcription. For those eukaryotic genes that contain introns, splicing is usually needed to create an mRNA molecule that can be translated into protein. For many eukaryotic introns, splicing occurs in a series of reactions which are catalyzed by the spliceosome, a complex of small nuclear ribonucleoproteins ( snRNPs). There exist self-splicing introns, that is, ribozymes that can catalyze their own excision from their parent RNA molecule. The process of transcription, splicing and translation is called gene expression, the central dogma of molecular biology. Splicing pathways Several methods of RNA spli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specificity (biochemistry)
Chemical specificity is the ability of binding site of a macromolecule (such as a protein) to bind specific ligands. The fewer ligands a protein can bind, the greater its specificity. Specificity describes the strength of binding between a given protein and ligand. This relationship can be described by a dissociation constant, which characterizes the balance between bound and unbound states for the protein-ligand system. In the context of a single enzyme and a pair of binding molecules, the two ligands can be compared as stronger or weaker ligands (for the enzyme) on the basis of their dissociation constants. (A lower value corresponds to a stronger binding.) Specificity for a set of ligands is unrelated to the ability of an enzyme to catalyze a given reaction, with the ligand as a substrate. If a given enzyme has a high chemical specificity, this means that the set of ligands to which it binds is limited, such that neither binding events nor catalysis can occur at an apprecia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Directionality (molecular Biology)
Directionality, in molecular biology and biochemistry, is the end-to-end chemical orientation of a single strand of nucleic acid. In a single strand of DNA or RNA, the chemical convention of naming carbon atoms in the nucleotide pentose-sugar-ring means that there will be a 5′ end (usually pronounced "five-prime end"), which frequently contains a phosphate group attached to the 5′ carbon of the ribose ring, and a 3′ end (usually pronounced "three-prime end"), which typically is unmodified from the ribose -OH substituent. In a DNA double helix, the strands run in opposite directions to permit base pairing between them, which is essential for replication or transcription of the encoded information. Nucleic acids can only be synthesized in vivo in the 5′-to-3′ direction, as the polymerases that assemble various types of new strands generally rely on the energy produced by breaking nucleoside triphosphate bonds to attach new nucleoside monophosphates to the 3′- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the ''S'' configuration. The human body cannot synthesize lysine. It is essential in humans and must therefore be obtained from the diet. In organisms that synthesise lysine, two main biosynthetic pathways exist, the diaminopimelate and α-aminoadipate pathways, which employ distinct e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the deprotonated –COO− form under biological conditions), and an imidazole side chain (which is partially protonated), classifying it as a positively charged amino acid at physiological pH. Initially thought essential only for infants, it has now been shown in longer-term studies to be essential for adults also. It is encoded by the codons CAU and CAC. Histidine was first isolated by Albrecht Kossel and Sven Gustaf Hedin in 1896. It is also a precursor to histamine, a vital inflammatory agent in immune responses. The acyl radical is histidyl. Properties of the imidazole side chain The conjugate acid (protonated form) of the imidazole side chain in histidine has a p''K''a of approximately 6.0. Thus, below a pH of 6, the imidazole ring ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |