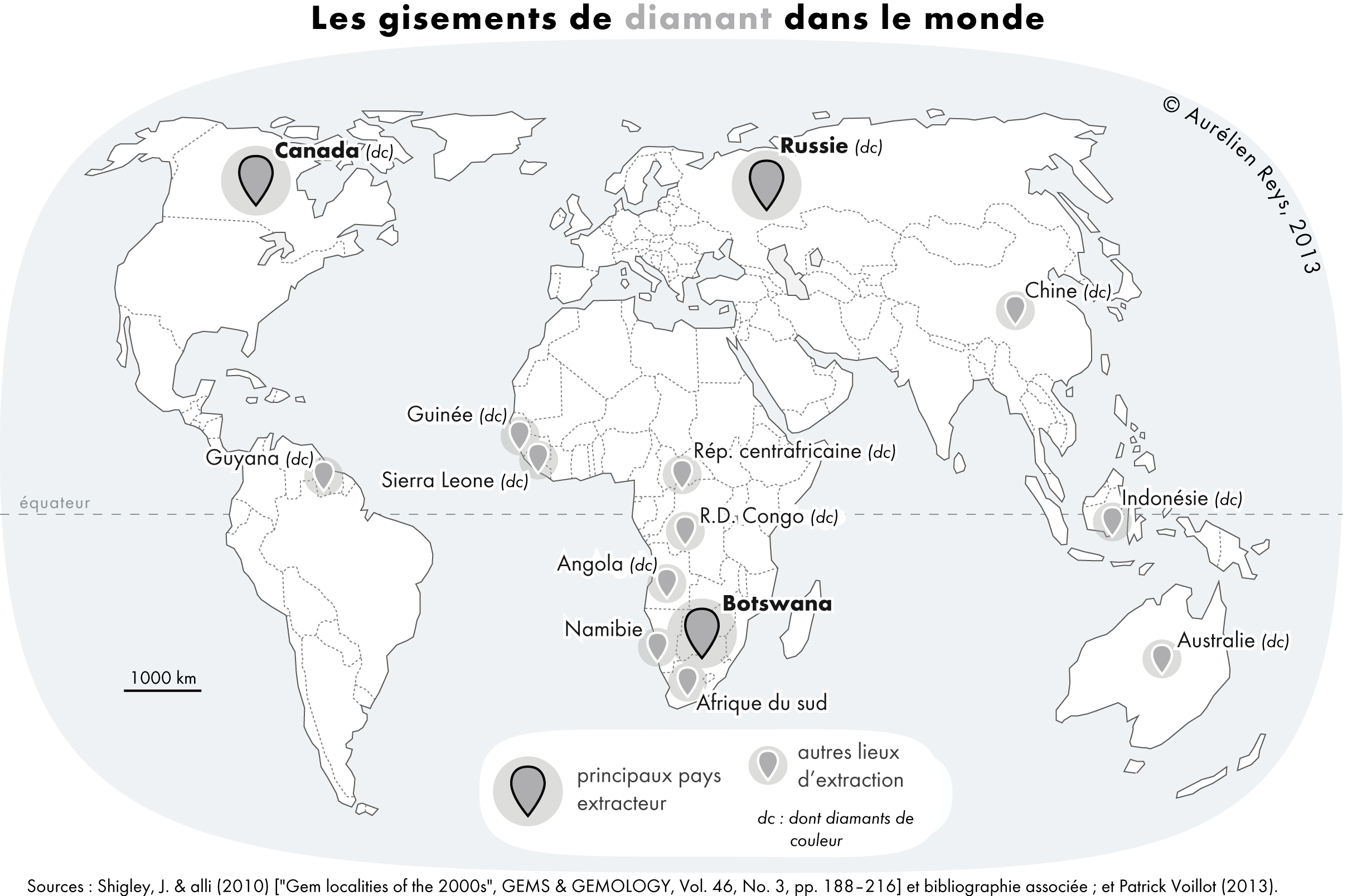
|
Superdense Carbon Allotropes
Superdense carbon allotropes are proposed configurations of carbon atoms that result in a stable material with a higher density than diamond. Few hypothetical carbon allotropes denser than diamond are known. All these allotropes can be divided at two groups: the first are hypothetically stable at ambient conditions; the second are high-pressure carbon allotropes which become quasi-stable only at high pressure. According to the SACADA database, the first group comprises the structures, called hP3, tI12, st12, r8, I41/a, P41212, m32, m32*, t32, t32*, H-carbon and uni. Among them st12 carbon was proposed as far as 1987 in the work of R. Biswas et al. MP8, OP8, SC4, BC-8 and (9,0) carbon allotropes belong to the second group - they are hypothetically quasi-stable at the high pressure. BC-8 carbon is not only a superdense allotrope but also one of the oldest hypothetical carbon structures - initially it was proposed in 1984 in the work R. Biswas et al. The MP8 structure proposed in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Atoms
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three isotopes occur naturally, C and C being stable, while C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity. Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth, enables this element to serve as a common element of all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen. The atoms of carbon can bond t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the Chemical stability, chemically stable form of carbon at Standard conditions for temperature and pressure, room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest Scratch hardness, hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth. Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of lattice defect, defects or impurities (about one per million of lattice atoms) color diamond blue (bor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Band Gap
In solid-state physics, a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference (in electron volts) between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote a valence electron bound to an atom to become a conduction electron, which is free to move within the crystal lattice and serve as a charge carrier to conduct electric current. It is closely related to the HOMO/LUMO gap in chemistry. If the valence band is completely full and the conduction band is completely empty, then electrons cannot move within the solid because there are no available states. If the electrons are not free to move within the crystal lattice, then there is no generated current due to no net charge carrier mobility. However, if some electrons transfer from th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |