|
Sulphonate
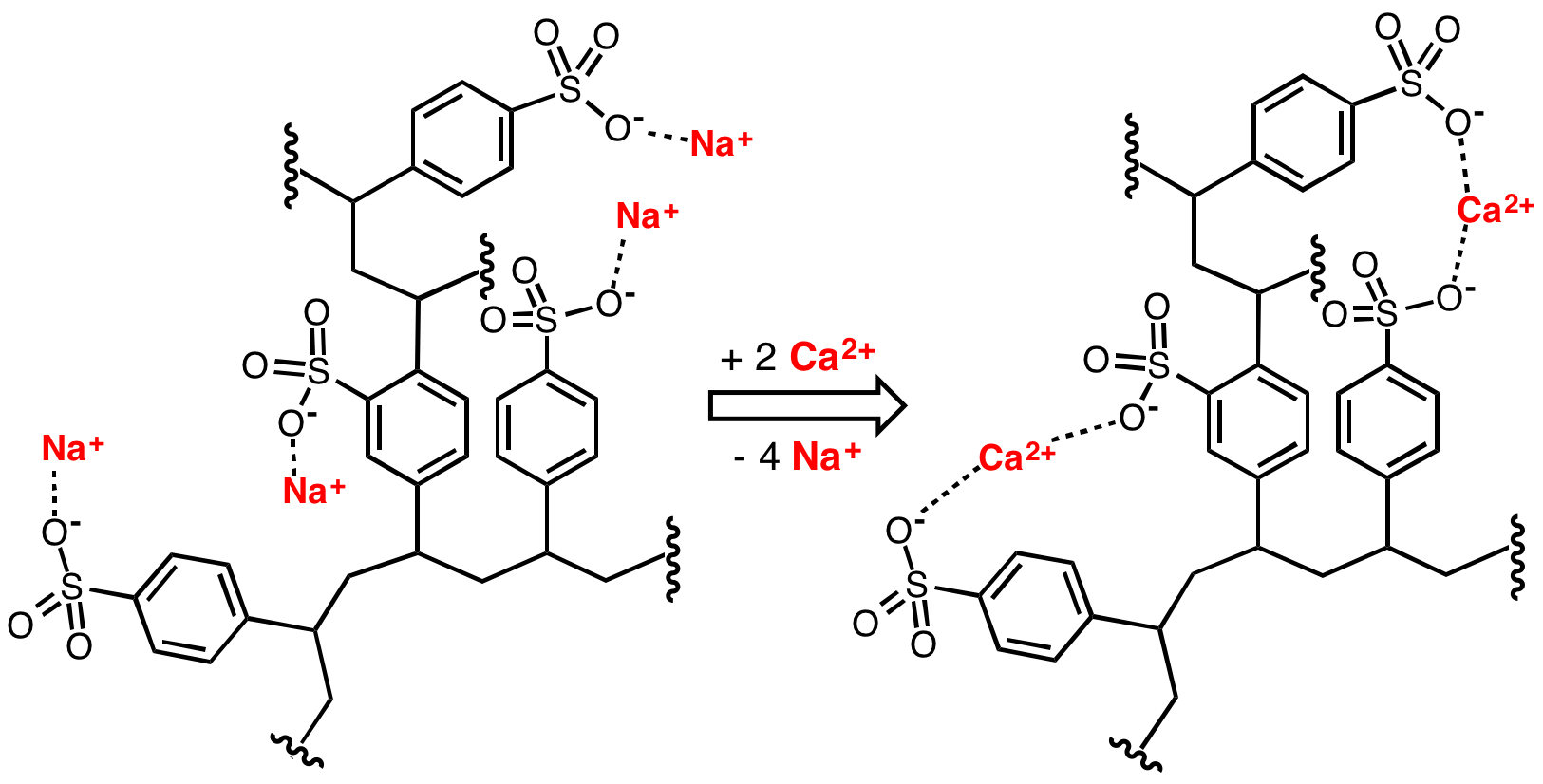
In organosulfur chemistry, a sulfonate is a salt or ester of a sulfonic acid. It contains the functional group , where R is an organic group. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, non-oxidizing, and colorless. Many useful compounds and even some biochemicals feature sulfonates. Sulfonate salts Anions with the general formula are called sulfonates. They are the conjugate bases of sulfonic acids with formula . As sulfonic acids tend to be strong acids, the corresponding sulfonates are weak bases. Due to the stability of sulfonate anions, the cations of sulfonate salts such as scandium triflate have application as Lewis acids. A classic preparation of sulfonates is the Strecker sulfite alkylation, in which an alkali sulfite salt displaces a halide, typically in the presence of an iodine catalyst: :RX + M2SO3 -> RSO3M + MX An alternative is the condensation of a sulfonyl halide with an alcohol in pyridine: :R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leaving Group
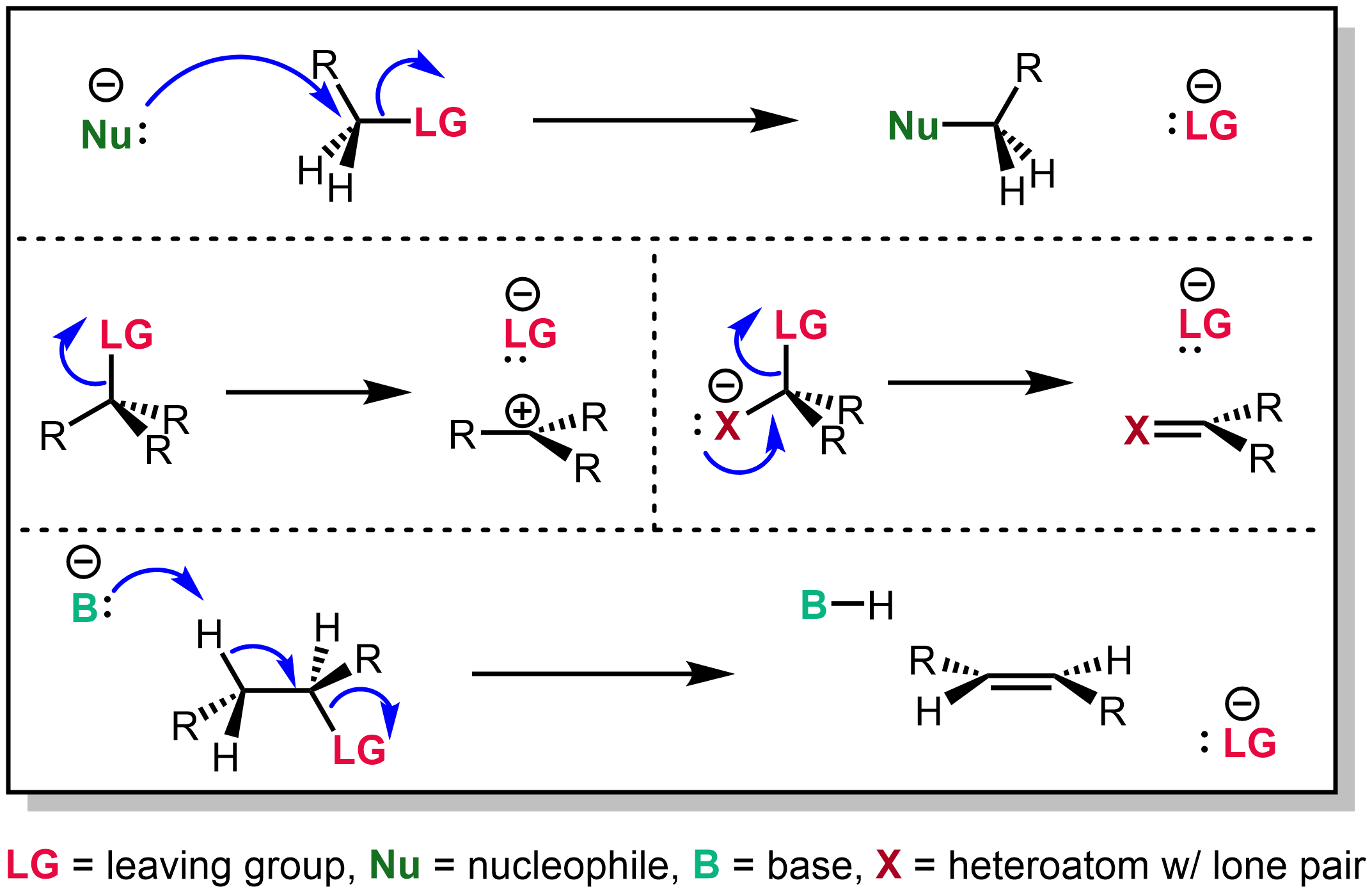
In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited to a fragment that departs with a pair of electrons in heterolytic bond cleavage. In this usage, a leaving group is a less formal but more commonly used synonym of the term '' nucleofuge''. In this context, leaving groups are generally anions or neutral species, departing from a neutral or cationic substrates, respectively, though in rare cases, cations leaving from a dicationic substrate are also known. A species' ability to serve as a leaving group depends on its ability to stabilize the additional electron density that results from bond heterolysis. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters such as tosylate (TsO−), while water (H2O), alcohols (HOR), and amines (R3N) are common neu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonate Anion Tet
In organosulfur chemistry, a sulfonate is a salt or ester of a sulfonic acid. It contains the functional group , where R is an organic group. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, non-oxidizing, and colorless. Many useful compounds and even some biochemicals feature sulfonates. Sulfonate salts Anions with the general formula are called sulfonates. They are the conjugate bases of sulfonic acids with formula . As sulfonic acids tend to be strong acids, the corresponding sulfonates are weak bases. Due to the stability of sulfonate anions, the cations of sulfonate salts such as scandium triflate have application as Lewis acids. A classic preparation of sulfonates is the Strecker sulfite alkylation, in which an alkali sulfite salt displaces a halide, typically in the presence of an iodine catalyst: :RX + M2SO3 -> RSO3M + MX An alternative is the condensation of a sulfonyl halide with an alcohol in pyridine: :ROH + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid ( sulfurous acid) is elusive, its salts are widely used. Sulfites are substances that naturally occur in some foods and the human body. They are also used as regulated food additives. When in food or drink, sulfites are often lumped together with sulfur dioxide.SeREGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL/ref> Structure The structure of the sulfite anion can be described with three equivalent resonance structures. In each resonance structure, the sulfur atom is double-bonded to one oxygen atom with a formal charge of zero (neutral), and sulfur is singly bonded to the other two oxygen atoms, which each carry a formal charge of −1, together accounting for the −2 charge on the anion. There is also a non-bonded lone pair on the sulfur, so the st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zonisamide
Zonisamide, sold under the brand name Zonegran among others, is a medication used to treat the symptoms of epilepsy and Parkinson's disease. Chemically it is a sulfonamide. It serves as an anticonvulsant used primarily as an adjunctive therapy in adults with Parkinson's disease, partial-onset seizures; infantile spasm, mixed seizure types of Lennox–Gastaut syndrome, myoclonic and generalized tonic clonic seizure. Despite this it is also sometimes used as a monotherapy for partial-onset seizures. In 2020, it was the 276th most commonly prescribed medication in the United States, with more than 1million prescriptions. Medical uses Epilepsy Zonisamide is approved in the United States, and United Kingdom for adjunctive treatment of partial seizures in adults and Japan for both adjunctive and monotherapy for partial seizures (simple, complex, secondarily generalized), generalized (tonic, tonic-clonic (grand mal), and atypical absence) and combined seizures. In Australia it is m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxime
In organic chemistry, an oxime is a organic compound belonging to the imines, with the general formula , where R is an organic side-chain and R’ may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides () with general structure . Oximes are usually generated by the reaction of hydroxylamine with aldehydes () or ketones (). The term ''oxime'' dates back to the 19th century, a combination of the words ''oxygen'' and ''imine''. Structure and properties If the two side-chains on the central carbon are different from each other—either an aldoxime, or a ketoxime with two different "R" groups—the oxime can often have two different geometric stereoisomeric forms according to the ''E''/''Z'' configuration. An older terminology of ''syn'' and ''anti'' was used to identify especially aldoximes according to whether the R group was closer or further from the hy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,4-Butane Sultone
1,4-butane sultone is a six-membered δ-sultone and the cyclic ester of 4-hydroxybutanesulfonic acid. As a sulfo-alkylating agent, 1,4-butanesultone is used to introduce the sulfobutyl group (–(CH2)4–SO3−) into hydrophobic compounds possessing nucleophilic functional groups, for example hydroxy groups (as in the case of β-cyclodextrin) or amino groups (as in the case of polymethine dyes). In such, the sulfobutyl group is present as neutral sodium salt and considerably increases the water solubility of the derivatives. Preparation A lab scale synthesis of 1,4-butanesultone starts from 4,4'-dichlorodibutyl ether (accessible from tetrahydrofuran treated with phosphorus oxychloride and concentrated sulfuric acid), which reacts with sodium sulfite forming the corresponding 4,4'-butanedisulfonic disodium salt. By passing it through an acidic ion exchanger, the disodium salt is converted into the disulphonic acid which forms two molecules of 1,4-butanesultone at elevated tem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propane-1,3-sultone
1,3-Propane sultone is the organosulfur compound with the formula (CH2)3SO3. It is a cyclic sulfonate ester, a class of compounds called sultones. It is a readily melting colorless solid. Synthesis It may be prepared by the acid catalyzed reaction of allyl alcohol and sodium bisulfite. Reactions 1,3-propane sultone is an activated ester and is susceptible to nucleophilic attack. It hydrolyzes to the acid. : It has been used in the synthesis of specialist surfactants, such as CHAPS detergent. Safety Typical of activated esters, 1,3-propane sultone is an alkylating agent. 1,3-Propane sultone is toxic, carcinogenic, mutagenic, and teratogenic. See also * 1,4-Butane sultone * Dimethyl sulfate * Vinylsulfonic acid * Isethionic acid * Sulfolane Sulfolane (also ''tetramethylene sulfone'', systematic name: 1λ6-thiolane-1,1-dione) is an organosulfur compound, formally a cyclic sulfone, with the formula (CH2)4SO2. It is a colorless liquid commonly used in the chemical industry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Triflate
Methyl trifluoromethanesulfonate, also commonly called methyl triflate and abbreviated MeOTf, is the organic compound with the formula CF3SO2OCH3. It is a colourless liquid which finds use in organic chemistry as a powerful methylating agent. The compound is closely related to methyl fluorosulfonate (FSO2OCH3). Although there has yet to be a reported human fatality, several cases were reported for methyl fluorosulfonate (LC50 (rat, 1 h) = 5 ppm), and methyl triflate is expected to have similar toxicity based on available evidence. Synthesis Methyl triflate is commercially available, however it may also be prepared in the laboratory by treating dimethyl sulfate with triflic acid. :CF3SO2OH + (CH3O)2SO2 → CF3SO2OCH3 + CH3OSO2OH Reactivity Hydrolysis Upon contact with water, methyl triflate loses its methyl group, forming triflic acid and methanol: :CF3SO2OCH3 + H2O → CF3SO2OH + CH3OH Methylation One ranking of methylating agents is (CH3)3O+ > CF3SO2OCH3 ≈ FSO2OC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Trifluoromethanesulfonate
Methyl trifluoromethanesulfonate, also commonly called methyl triflate and abbreviated MeOTf, is the organic compound with the formula CF3SO2OCH3. It is a colourless liquid which finds use in organic chemistry as a powerful methylating agent. The compound is closely related to methyl fluorosulfonate (FSO2OCH3). Although there has yet to be a reported human fatality, several cases were reported for methyl fluorosulfonate (LC50 (rat, 1 h) = 5 ppm), and methyl triflate is expected to have similar toxicity based on available evidence. Synthesis Methyl triflate is commercially available, however it may also be prepared in the laboratory by treating dimethyl sulfate with triflic acid. :CF3SO2OH + (CH3O)2SO2 → CF3SO2OCH3 + CH3OSO2OH Reactivity Hydrolysis Upon contact with water, methyl triflate loses its methyl group, forming triflic acid and methanol: :CF3SO2OCH3 + H2O → CF3SO2OH + CH3OH Methylation One ranking of methylating agents is (CH3)3O+ > CF3SO2OCH3 ≈ FSO2OCH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For example, protonation of methanol gives ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |