|
Sulfonamide
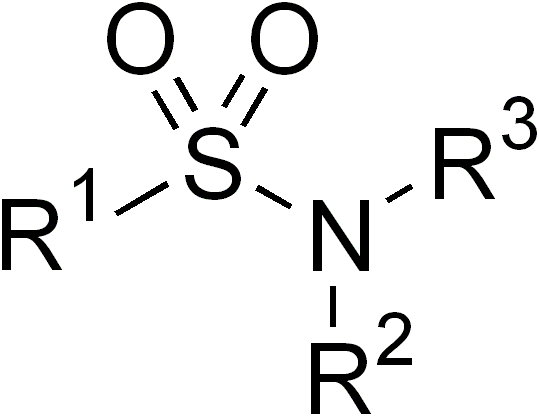
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonamide (medicine)
Sulfonamide is a functional group (a part of a molecule) that is the basis of several groups of drugs, which are called sulphonamides, sulfa drugs or sulpha drugs. The original antibacterial sulfonamides are synthetic (nonantibiotic) antimicrobial agents that contain the sulfonamide group. Some sulfonamides are also devoid of antibacterial activity, e.g., the anticonvulsant sultiame. The sulfonylureas and thiazide diuretics are newer drug groups based upon the antibacterial sulfonamides. Allergies to sulfonamides are common. The overall incidence of adverse drug reactions to sulfa antibiotics is approximately 3%, close to penicillin; hence medications containing sulfonamides are prescribed carefully. Sulfonamide drugs were the first broadly effective antibacterials to be used systemically, and paved the way for the antibiotic revolution in medicine. Function In bacteria, antibacterial sulfonamides act as competitive inhibitors of the enzyme dihydropteroate synthas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfamethoxazole
Sulfamethoxazole (SMZ or SMX) is an antibiotic. It is used for bacterial infections such as urinary tract infections, bronchitis, and prostatitis and is effective against both gram negative and positive bacteria such as ''Listeria monocytogenes'' and '' E. coli''. Common side effects include nausea, vomiting, loss of appetite, and skin rashes. It is a sulfonamide and bacteriostatic. It resembles a component of folic acid. It prevents folic acid synthesis in the bacteria that must synthesize their own folic acid. Mammalian cells, and some bacteria, do not synthesize but require preformed folic acid (vitamin B9); they are therefore insensitive to sulfamethoxazole. It was introduced to the United States in 1961. It is now mostly used in combination with trimethoprim (abbreviated SMX-TMP). The SMX-TMP combination is on the WHO Model List of Essential medicines as a first-choice treatment for urinary tract infections. Other names include: sulfamethalazole, sulfisomezole,PubChem" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sultam
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonamide
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfanilamide
Sulfanilamide (also spelled sulphanilamide) is a sulfonamide antibacterial drug. Chemically, it is an organic compound consisting of an aniline derivatized with a sulfonamide group. Powdered sulfanilamide was used by the Allies in World War II to reduce infection rates and contributed to a dramatic reduction in mortality rates compared to previous wars. Sulfanilamide is rarely if ever used systemically due to toxicity and because more effective sulfonamides are available for this purpose. Modern antibiotics have supplanted sulfanilamide on the battlefield; however, sulfanilamide remains in use today in the form of topical preparations, primarily for treatment of vaginal yeast infections mainly vulvovaginitis which is caused by ''Candida albicans''. The term "sulfanilamides" is also sometimes used to describe a family of molecules containing these functional groups. Examples include: * Furosemide, a loop diuretic * Sulfadiazine, an antibiotic * Sulfamethoxazole, an antibioti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tosyl Chloride
4-Toluenesulfonyl chloride (''p''-toluenesulfonyl chloride, toluene-''p''-sulfonyl chloride) is an organic compound with the formula CH3C6H4SO2Cl. This white, malodorous solid is a reagent widely used in organic synthesis. Abbreviated TsCl or TosCl, it is a derivative of toluene and contains a sulfonyl chloride (−SO2Cl) functional group. Uses In characteristic manner, TsCl converts alcohols (abbreviated ROH) into the corresponding toluenesulfonate esters, or tosyl derivatives ("tosylates"): : CH3C6H4SO2Cl + ROH → CH3C6H4SO2OR + HCl Tosylates can be cleaved with lithium aluminium hydride: : 4 CH3C6H4SO2OR + LiAlH4 → LiAl(O3SC6H4CH3)4 + 4 RH Thus, tosylation followed by reduction allows for removal of a hydroxyl group. Likewise, TsCl is used to prepare sulfonamides from amines: :CH3C6H4SO2Cl + R2NH → CH3C6H4SO2NR2 + HCl The resulting sulfonamides are non-basic and, when derived from primary amines, are even acidic. TsCl reacts with hydrazine to give ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substitue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy, treatment and antibiotic prophylaxis, prevention of such infections. They may either bactericide, kill or bacteriostatic agent, inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the common cold or influenza; drugs which inhibit viruses are termed antiviral drugs or antivirals rather than antibiotics. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek language, Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against microbes, but in the usual medical usage, antibiotics (such as penicillin) are those produced naturally (by one microorgani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saccharin
Saccharin (''aka'' saccharine, Sodium sacchari) is an artificial sweetener with effectively no nutritional value. It is about 550 times as sweet as sucrose but has a bitter or metallic aftertaste, especially at high concentrations. Saccharin is used to sweeten products such as drinks, candies, cookies, and especially for masking bitter taste of some medicines. Etymology Saccharin derives its name from the word "saccharine", meaning "sugary". The word saccharine is used figuratively, often in a derogative sense, to describe something "unpleasantly over-polite" or "overly sweet". Both words are derived from the Greek word (''sakkharon'') meaning "gravel". Similarly, saccharose is an obsolete name for sucrose (table sugar). Properties Saccharin is heat-stable. It does not react chemically with other food ingredients; as such, it stores well. Blends of saccharin with other sweeteners are often used to compensate for each sweetener's weaknesses and faults. A 10:1 cyclamate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
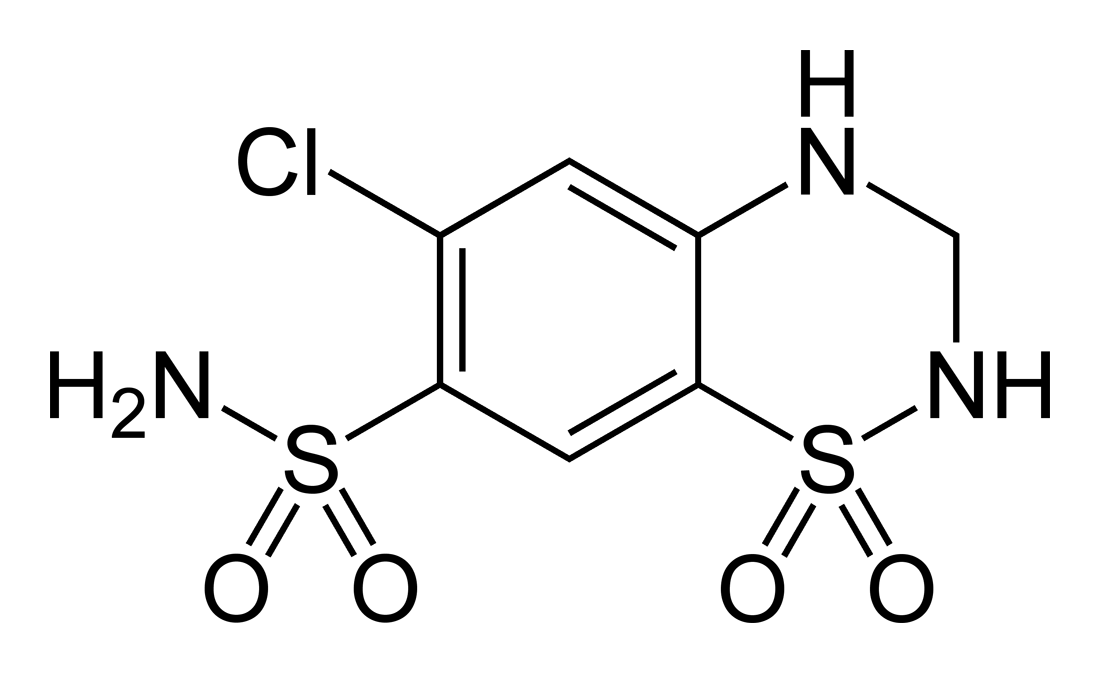
Sulthiame
Sultiame (or sulthiame) is a sulfonamide and inhibitor of the enzyme carbonic anhydrase. It is used as an anticonvulsant. History Sultiame was first synthesised in the laboratories of Bayer AG in the mid 1950s and eventually launched as Ospolot in Europe and other markets the early 1960s. It never became a registered drug in the United States. The brand was transferred to Desitin GmbH in 1993 and is sold in several European countries, in Israel, Japan, and Australia. Sultiame became established as a second-line drug for treatment of partial epilepsy in the 1960s and 1970s and was often used in combination with the established anticonvulsant phenytoin. Temporal lobe seizures appeared particularly responsive to sultiame. Doubts subsequently arose as to whether sultiame has intrinsic anticonvulsant properties. After discovering sultiame's ability to raise the blood levels of phenytoin, it was assumed that sultiame would only act in combination with phenytoin. This finding, togethe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonic Acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, , a tautomer of sulfurous acid, . Salt (chemistry), Salts or esters of sulfonic acids are called sulfonates. Preparation Aryl sulfonic acids are produced by the process of sulfonation. Usually the sulfonating agent is sulfur trioxide. A large scale application of this method is the production of alkylbenzenesulfonic acids: :RC6H5 + SO3 -> RC6H4SO3H In this reaction, sulfur trioxide is an electrophile and the arene is the nucleophile. The reaction is an example of electrophilic aromatic substitution. Alkyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medicine
Medicine is the science and Praxis (process), practice of caring for a patient, managing the diagnosis, prognosis, Preventive medicine, prevention, therapy, treatment, Palliative care, palliation of their injury or disease, and Health promotion, promoting their health. Medicine encompasses a variety of health care practices evolved to maintain and restore health by the prevention (medical), prevention and therapy, treatment of illness. Contemporary medicine applies biomedical sciences, biomedical research, medical genetics, genetics, and medical technology to diagnosis (medical), diagnose, treat, and prevent injury and disease, typically through pharmaceuticals or surgery, but also through therapies as diverse as psychotherapy, splint (medicine), external splints and traction, medical devices, biologic medical product, biologics, and Radiation (medicine), ionizing radiation, amongst others. Medicine has been practiced since Prehistoric medicine, prehistoric times, and for most o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |