|
Sugars In Wine
Sugars in wine are at the heart of what makes winemaking possible. During the process of fermentation, sugars from wine grapes are broken down and converted by yeast into alcohol (ethanol) and carbon dioxide. Grapes accumulate sugars as they grow on the grapevine through the translocation of sucrose molecules that are produced by photosynthesis from the leaves. During ripening the sucrose molecules are hydrolyzed (separated) by the enzyme invertase into glucose and fructose. By the time of harvest, between 15 and 25% of the grape will be composed of simple sugars. Both glucose and fructose are six-carbon sugars but three-, four-, five- and seven-carbon sugars are also present in the grape. Not all sugars are fermentable, with sugars like the five-carbon arabinose, rhamnose and xylose still being present in the wine after fermentation. Very high sugar content will effectively kill the yeast once a certain (high) alcohol content is reached. For these reasons, no wine is ever fermen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grape Slice
A grape is a fruit, botanically a berry, of the deciduous woody vines of the flowering plant genus ''Vitis''. Grapes are a non- climacteric type of fruit, generally occurring in clusters. The cultivation of grapes began perhaps 8,000 years ago, and the fruit has been used as human food over history. Eaten fresh or in dried form (as raisins, currants and sultanas), grapes also hold cultural significance in many parts of the world, particularly for their role in winemaking. Other grape-derived products include various types of jam, juice, vinegar and oil. History The Middle East is generally described as the homeland of grape and the cultivation of this plant began there 6,000–8,000 years ago. Yeast, one of the earliest domesticated microorganisms, occurs naturally on the skins of grapes, leading to the discovery of alcoholic drinks such as wine. The earliest archeological evidence for a dominant position of wine-making in human culture dates from 8,000 years ago in Georg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Simple Sugar
Monosaccharides (from Greek '' monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units ( monomers) from which all carbohydrates are built. They are usually colorless, water-soluble, and crystalline solids. Contrary to their name (sugars), only some monosaccharides have a sweet taste. Most monosaccharides have the formula (though not all molecules with this formula are monosaccharides). Examples of monosaccharides include glucose (dextrose), fructose (levulose), and galactose. Monosaccharides are the building blocks of disaccharides (such as sucrose and lactose) and polysaccharides (such as cellulose and starch). The table sugar used in everyday vernacular is itself a disaccharide sucrose comprising one molecule of each of the two monosaccharides D-glucose and D-fructose. Each carbon atom that supports a hydroxyl group is chiral, except those at the end of the chain. This gives rise to a number of isomeri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosidic Bond
A glycosidic bond or glycosidic linkage is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate. A glycosidic bond is formed between the hemiacetal or hemiketal group of a saccharide (or a molecule derived from a saccharide) and the hydroxyl group of some compound such as an alcohol. A substance containing a glycosidic bond is a glycoside. The term 'glycoside' is now extended to also cover compounds with bonds formed between hemiacetal (or hemiketal) groups of sugars and several chemical groups other than hydroxyls, such as -SR (thioglycosides), -SeR (selenoglycosides), -NR1R2 (N-glycosides), or even -CR1R2R3 (C-glycosides). Particularly in naturally occurring glycosides, the compound ROH from which the carbohydrate residue has been removed is often termed the aglycone, and the carbohydrate residue itself is sometimes referred to as the 'glycone'. S-, N-, C-, and O-glycosidic bonds Glyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Invertase
Invertase is an enzyme that catalyzes the hydrolysis (breakdown) of sucrose (table sugar) into fructose and glucose. Alternative names for invertase include , saccharase, glucosucrase, beta-h-fructosidase, beta-fructosidase, invertin, sucrase, maxinvert L 1000, fructosylinvertase, alkaline invertase, acid invertase, and the systematic name: beta-fructofuranosidase. The resulting mixture of fructose and glucose is called inverted sugar syrup. Related to invertases are sucrases. Invertases and sucrases hydrolyze sucrose to give the same mixture of glucose and fructose. Invertase is a glycoprotein that hydrolyses (cleaves) the non-reducing terminal beta-fructofuranoside residues. Thus, its systematic name is beta-fructofuranosidase. Invertases cleave the O-C(fructose) bond, whereas the sucrases cleave the O-C(glucose) bond. Invertase cleaves the alpha-1,2-glycosidic bond of sucrose. For industrial use, invertase is usually derived from yeast. It is also synthesized by bees, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monosaccharides
Monosaccharides (from Greek '' monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built. They are usually colorless, water-soluble, and crystalline solids. Contrary to their name (sugars), only some monosaccharides have a sweet taste. Most monosaccharides have the formula (though not all molecules with this formula are monosaccharides). Examples of monosaccharides include glucose (dextrose), fructose (levulose), and galactose. Monosaccharides are the building blocks of disaccharides (such as sucrose and lactose) and polysaccharides (such as cellulose and starch). The table sugar used in everyday vernacular is itself a disaccharide sucrose comprising one molecule of each of the two monosaccharides D-glucose and D-fructose. Each carbon atom that supports a hydroxyl group is chiral, except those at the end of the chain. This gives rise to a number of isomeric for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disaccharide
A disaccharide (also called a double sugar or ''biose'') is the sugar formed when two monosaccharides are joined by glycosidic linkage. Like monosaccharides, disaccharides are simple sugars soluble in water. Three common examples are sucrose, lactose, and maltose. Disaccharides are one of the four chemical groupings of carbohydrates ( monosaccharides, disaccharides, oligosaccharides, and polysaccharides). The most common types of disaccharides—sucrose, lactose, and maltose—have 12 carbon atoms, with the general formula C12H22O11. The differences in these disaccharides are due to atomic arrangements within the molecule. The joining of monosaccharides into a double sugar happens by a condensation reaction, which involves the elimination of a water molecule from the functional groups only. Breaking apart a double sugar into its two monosaccharides is accomplished by hydrolysis with the help of a type of enzyme called a disaccharidase. As building the larger sugar ejects ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chaptalization
Chaptalization is the process of adding sugar to unfermented grape must in order to increase the alcohol content after fermentation. The technique is named after its developer, the French chemist Jean-Antoine-Claude Chaptal. This process is not intended to make the wine sweeter, but rather to provide more sugar for the yeast to ferment into alcohol. Chaptalization has generated controversy and discontent in the French wine industry due to advantages that the process is perceived to give producers in poor-climate areas. In response to violent demonstrations by protesters in 1907, the French government began regulating the amount of sugar that can be added to wine. Chaptalization is sometimes referred to as enrichment, for example in the European Union wine regulations specifying the legality of the practice within EU. The legality of chaptalization varies by country, region, and even wine type. In general, it is legal in regions that produce grapes with low sugar content, such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Body (wine)
The use of wine tasting descriptors allows the taster to qualitatively relate the aromas and flavors that the taster experiences and can be used in assessing the overall quality of wine. Wine writers differentiate wine tasters from casual enthusiasts; tasters attempt to give an objective description of the wine's taste (often taking a systematic approach to tasting), casual enthusiasts appreciate wine but pause their examination sooner than tasters. The primary source of a person's ability to taste wine is derived from their olfactory senses. A taster's own personal experiences play a significant role in conceptualizing what they are tasting and attaching a description to that perception. The individual nature of tasting means that descriptors may be perceived differently among various tasters. The following is an incomplete list of wine tasting descriptors and a common meaning of the terms. These terms and usage are from Karen MacNeil's 2001 edition of ''The Wine Bible'' u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol Content
Alcohol by volume (abbreviated as ABV, abv, or alc/vol) is a standard measure of how much alcohol (ethanol) is contained in a given volume of an alcoholic beverage (expressed as a volume percent). It is defined as the number of millilitres (mL) of pure ethanol present in of solution at . The number of millilitres of pure ethanol is the mass of the ethanol divided by its density at , which is . The ABV standard is used worldwide. The International Organization of Legal Metrology has tables of density of water–ethanol mixtures at different concentrations and temperatures. In some countries, e.g. France, alcohol by volume is often referred to as degrees Gay-Lussac (after the French chemist Joseph Louis Gay-Lussac), although there is a slight difference since the Gay-Lussac convention uses the International Standard Atmosphere value for temperature, . Volume change Mixing two solutions of alcohol of different strengths usually causes a change in volume. Mixing pure water with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Residual Sugar
The subjective sweetness of a wine is determined by the interaction of several factors, including the amount of sugar in the wine, but also the relative levels of alcohol, acids, and tannins. Sugars and alcohol enhance a wine's sweetness, while acids cause sourness and bitter tannins cause bitterness. These principles are outlined in the 1987 work by Émile Peynaud, ''The Taste of Wine''. History ''Vintage: The Story of Wine'', a book authored by British wine writer Hugh Johnson, presents several methods that have been used throughout history to sweeten wine. The most common way was to harvest the grapes as late as possible. This method was advocated by Virgil and Martial in Roman times. In contrast, the ancient Greeks would harvest the grapes early, to preserve some of their acidity, and then leave them in the sun for a few days to allow them to shrivel and concentrate the sugar. In Crete, a similar effect was achieved by twisting the stalks of the grape to deprive the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dry (wine)
The use of wine tasting descriptors allows the taster to qualitatively relate the aromas and flavors that the taster experiences and can be used in assessing the overall quality of wine. Wine writers differentiate wine tasters from casual enthusiasts; tasters attempt to give an objective description of the wine's taste (often taking a systematic approach to tasting), casual enthusiasts appreciate wine but pause their examination sooner than tasters. The primary source of a person's ability to taste wine is derived from their olfactory senses. A taster's own personal experiences play a significant role in conceptualizing what they are tasting and attaching a description to that perception. The individual nature of tasting means that descriptors may be perceived differently among various tasters. The following is an incomplete list of wine tasting descriptors and a common meaning of the terms. These terms and usage are from Karen MacNeil's 2001 edition of ''The Wine Bible'' un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
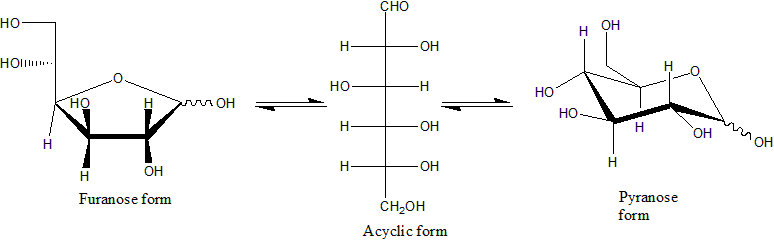
Xylose
Xylose ( grc, ξύλον, , "wood") is a sugar first isolated from wood, and named for it. Xylose is classified as a monosaccharide of the aldopentose type, which means that it contains five carbon atoms and includes an aldehyde functional group. It is derived from hemicellulose, one of the main constituents of biomass. Like most sugars, it can adopt several structures depending on conditions. With its free aldehyde group, it is a reducing sugar. Structure The acyclic form of xylose has chemical formula . The cyclic hemiacetal isomers are more prevalent in solution and are of two types: the pyranoses, which feature six-membered rings, and the furanoses, which feature five-membered rings (with a pendant group). Each of these rings is subject to further isomerism, depending on the relative orientation of the anomeric hydroxy group. The dextrorotary form, -xylose, is the one that usually occurs endogenously in living things. A levorotary form, -xylose, can be synthe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |