|
Sterol Regulatory Element-binding Protein
Sterol regulatory element-binding proteins (SREBPs) are transcription factors that bind to the sterol regulatory element DNA sequence TCACNCCAC. Mammalian SREBPs are encoded by the genes ''SREBF1'' and ''SREBF2''. SREBPs belong to the basic-helix-loop-helix leucine zipper class of transcription factors. Unactivated SREBPs are attached to the nuclear envelope and endoplasmic reticulum membranes. In cells with low levels of sterols, SREBPs are cleaved to a water-soluble N-terminal domain that is translocated to the nucleus. These activated SREBPs then bind to specific sterol regulatory element DNA sequences, thus upregulating the synthesis of enzymes involved in sterol biosynthesis. Sterols in turn inhibit the cleavage of SREBPs and therefore synthesis of additional sterols is reduced through a negative feed back loop. Isoforms Mammalian genomes have two separate SREBP genes ( and ): * SREBP-1 expression produces two different isoforms, SREBP-1a and -1c. These isoforms differ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information. Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic, and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among various mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MRNA
In molecular biology, messenger ribonucleic acid (mRNA) is a single-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and is read by a ribosome in the process of Protein biosynthesis, synthesizing a protein. mRNA is created during the process of Transcription (biology), transcription, where an enzyme (RNA polymerase) converts the gene into primary transcript mRNA (also known as pre-mRNA). This pre-mRNA usually still contains introns, regions that will not go on to code for the final amino acid sequence. These are removed in the process of RNA splicing, leaving only exons, regions that will encode the protein. This exon sequence constitutes mature mRNA. Mature mRNA is then read by the ribosome, and, utilising amino acids carried by transfer RNA (tRNA), the ribosome creates the protein. This process is known as Translation (biology), translation. All of these processes form part of the central dogma of molecular biology, which describes the flow of genet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Annual Review Of Genetics
The ''Annual Review of Genetics'' is an annual peer-reviewed scientific review journal published by Annual Reviews. It was established in 1967 and covers all topics related to the genetics of viruses, bacteria, fungi, plants, and animals, including humans. The current editor is Tatjana Piotrowski. As of 2021, ''Journal Citation Reports'' gives the journal a 2020 impact factor of 16.830, ranking it fourth out of 175 journals in the category "Genetics & Heredity". History In 1965, the nonprofit publisher Annual Reviews surveyed geneticists to determine if there was a need for an annual journal that published review articles about recent developments in the field of genetics. Responses to the survey were favorable, with the first volume of the ''Annual Review of Genetics'' published two years later in 1967. Its inaugural editor was Herschel L. Roman. As of 2020, it was published both in print and electronically. It defines its scope as covering various aspects of genetics, includ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LDL Receptor
The low-density lipoprotein receptor (LDL-R) is a mosaic protein of 839 amino acids (after removal of 21-amino acid signal peptide) that mediates the endocytosis of cholesterol-rich low-density lipoprotein (LDL). It is a cell-surface receptor that recognizes apolipoprotein B100 (ApoB100), which is embedded in the outer phospholipid layer of very low-density lipoprotein (VLDL), their remnants—i.e. intermediate-density lipoprotein (IDL), and LDL particles. The receptor also recognizes apolipoprotein E (ApoE) which is found in chylomicron remnants and IDL. In humans, the LDL receptor protein is encoded by the gene on chromosome 19. It belongs to the low density lipoprotein receptor gene family. It is most significantly expressed in bronchial epithelial cells and adrenal gland and cortex tissue. Michael S. Brown and Joseph L. Goldstein were awarded the 1985 Nobel Prize in Physiology or Medicine for their identification of LDL-R and its relation to cholesterol metabolism ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eukaryotic Cells
Eukaryotes () are organisms whose Cell (biology), cells have a cell nucleus, nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the Three-domain system, three domains of life. Bacteria and Archaea (both prokaryotes) make up the other two domains. The eukaryotes are usually now regarded as having emerged in the Archaea or as a sister of the Asgard (archaea), Asgard archaea. This implies that there are only Two-domain system, two domains of life, Bacteria and Archaea, with eukaryotes incorporated among archaea. Eukaryotes represent a small minority of the number of organisms, but, due to their generally much larger size, their collective global biomass (ecology), biomass is estimated to be about equal to that of prokaryotes. Eukaryotes emerged approximately 2.3–1.8 billion years ago, during the Proterozoic eon, likely as Flagellated cell, flagellated phagotrophs. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SREBP Cleavage-activating Protein
Sterol regulatory element-binding protein cleavage-activating protein, also known as SREBP cleavage-activating protein or SCAP is a protein that in humans is encoded by the ''SCAP'' gene. SCAP contains a sterol-sensing domain (SSD) and seven WD domains. In cholesterol-depleted cells, this protein binds to sterol regulatory element binding proteins (SREBPs) and mediates their transport from the ER to the Golgi apparatus. The SREBPs are then proteolytically cleaved and stimulate sterol biosynthesis. Function SCAP is a regulatory protein that is required for the proteolytic cleavage of the sterol regulatory element-binding protein (SREBP). SCAP is an integral membrane protein located in the endoplasmic reticulum (ER). One of the cytosolic regions of SCAP contains a hexapeptide amino acid sequence, MELADL, that functions to detect cellular cholesterol. When cholesterol is present, SCAP undergoes a conformational change that prevents it from activating SREBP and cholesterol synth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane-bound Transcription Factor Peptidase, Site 2
Membrane-bound transcription factor site-2 protease, also known as S2P endopeptidase or site-2 protease (S2P), is an enzyme () encoded by the gene which liberates the N-terminal fragment of sterol regulatory element-binding protein (SREBP) transcription factors from membranes. S2P cleaves the transmembrane domain of SREPB, making it a member of the class of intramembrane proteases. S2P catalyses the following chemical reaction : Cleaves several transcription factors that are type-2 transmembrane proteins within membrane-spanning domains. Known substrates include sterol regulatory element-binding protein (SREBP)-1, SREBP-2 and forms of the transcriptional activator ATF6. This enzyme belongs to the peptidase family M50. Function This gene encodes an intramembrane zinc metalloprotease, which is essential in development. This protease functions in the signal protein activation involved in sterol control of transcription and the ER stress response. Mutations in this gene have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane-bound Transcription Factor Peptidase, Site 1
Membrane-bound transcription factor site-1 protease, or site-1 protease (S1P) for short, also known as subtilisin/kexin-isozyme 1 (SKI-1), is an enzyme (EC 3.4.21.112) that in humans is encoded by the MBTPS1 gene. S1P cleaves the endoplasmic reticulum loop of sterol regulatory element-binding protein (SREBP) transcription factors. Function This gene encodes a member of the subtilisin-like proprotein convertase family, which includes proteases that process protein and peptide precursors trafficking through regulated or constitutive branches of the secretory pathway. The encoded protein undergoes an initial autocatalytic processing event in the endoplasmic reticulum (ER) to generate a heterodimer which exits the ER and sorts to the cis/medial- Golgi where a second autocatalytic event takes place and the catalytic activity is acquired. It encodes a type 1 membrane bound protease which is ubiquitously expressed and regulates cholesterol or lipid homeostasis via cleavage of substrate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Hierarchy of proteases Based on catalytic residue Proteases can be classified into seven broad groups: * Serine protease ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling lif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
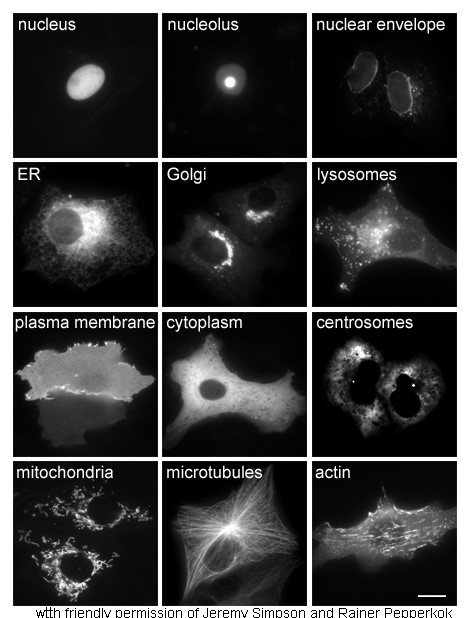
Cytoplasm
In cell biology, the cytoplasm is all of the material within a eukaryotic cell, enclosed by the cell membrane, except for the cell nucleus. The material inside the nucleus and contained within the nuclear membrane is termed the nucleoplasm. The main components of the cytoplasm are cytosol (a gel-like substance), the organelles (the cell's internal sub-structures), and various cytoplasmic inclusions. The cytoplasm is about 80% water and is usually colorless. The submicroscopic ground cell substance or cytoplasmic matrix which remains after exclusion of the cell organelles and particles is groundplasm. It is the hyaloplasm of light microscopy, a highly complex, polyphasic system in which all resolvable cytoplasmic elements are suspended, including the larger organelles such as the ribosomes, mitochondria, the plant plastids, lipid droplets, and vacuoles. Most cellular activities take place within the cytoplasm, such as many metabolic pathways including glycolysis, and proces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |