|
Squarate
Squaric acid, also called quadratic acid because its four carbon atoms approximately form a square, is a diprotic organic acid with the chemical formula C4O2(OH)2. The conjugate base of squaric acid is the hydrogensquarate anion ; and the conjugate base of the hydrogensquarate anion is the divalent squarate anion . This is one of the oxocarbon anions, which consist only of carbon and oxygen. Squaric acid is a reagent for chemical synthesis, used for instance to make photosensitive squaraine dyes and inhibitors of protein tyrosine phosphatases. Chemical properties Squaric acid is a white crystalline powder. The onset of thermal decomposition depends on the different thermodynamic conditions such as heating rates. The structure of squaric acid is not a perfect square, as the carbon–carbon bond lengths are not quite equal. The high acidity with p''K''a = 1.5 for the first proton and p''K''a = 3.4 for the second is attributable to resonance stabilization of the anion. Because t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudo-oxocarbon Anion
In chemistry, the term pseudo-oxocarbon anion is used to refer to a negative ion that is conceptually derived from an oxocarbon anion through replacement of one or more of the basic oxygen atoms by chemically similar elements or functional groups, such as sulfur (S), selenium (Se), or dicyanomethylene (=C(CN)2). Typical examples are the anions 2-(Dicyanomethylene)croconate, croconate violet, and croconate blue, derived from the croconate anion by replacing one, two, or three oxygen atoms by dicyanomethylene groups: These anions retain many of the properties of the parent, including the delocalized bond in the ring and the delocalized charge in the atoms attached to the ring. Alexander J. Fatiadi (1978), ''Synthesis of 1,3-(dicyanomethylene)croconate salts. New bond-delocalized dianion, "Croconate Violet"''. Journal of the American Chemical Society, volume 100 issue 8, pages 2586–2587. Alexander J. Fatiadi (1980), ''Pseudooxocarbons. Synthesis of 1,2,3-tris(dicyanomethy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylenediol
Acetylenediol, or ethynediol, is a chemical substance with formula HO−C≡C−OH (an ynol). It is the diol of acetylene. Acetylenediol is unstable in the condensed phase, although its tautomer glyoxal (CHO)2 is well known. Detection Acetylenediol was first observed in the gas-phase by mass spectrometry. The compound was later obtained by photolysis of squaric acid in a solid argon matrix at . Derivatives Alkoxide derivatives Like the diol, most simple ether derivatives are labile. Di-tert-butoxyacetylene is however a distillable liquid. Acetylenediolate salts Salts of the acetylenediolate (ethynediolate) dianion −O−C≡C−O− are known. They are not however prepared from ethynediol, but by the reduction of carbon monoxide. Potassium acetylenediolate (K2C2O2) was first obtained by Liebig in 1834, from the reaction of carbon monoxide with metallic potassium;Justus Liebig (1834), Annalen der Chemie und Pharmacie, volume 11, p. 182. Cited by Raymond N. Vrtis et a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxocarbon Anion
In chemistry, an oxocarbon anion is a negative ion consisting solely of carbon and oxygen atoms, and therefore having the general formula for some integers ''x'', ''y'', and ''n''. The most common oxocarbon anions are carbonate, , and oxalate, . There is however a large number of stable anions in this class, including several ones that have research or industrial use. There are also many unstable anions, like and , that have a fleeting existence during some chemical reactions; and many hypothetical species, like , that have been the subject of theoretical studies but have yet to be observed. Stable oxocarbon anions form salts with a large variety of cations. Unstable anions may persist in very rarefied gaseous state, such as in interstellar clouds. Most oxocarbon anions have corresponding moieties in organic chemistry, whose compounds are usually esters. Thus, for example, the oxalate moiety occurs in the ester dimethyl oxalate . Electronic structure of the carbonate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma-Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company that is owned by the German chemical conglomerate Merck Group. Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company and Aldrich Chemical Company. It grew through various acquisitions until it had over 9,600 employees and was listed on the Fortune 1000. The company is headquartered in St. Louis and has operations in approximately 40 countries. In 2015, the German chemical conglomerate Merck Group acquired Sigma-Aldrich for $17 billion. The company is currently a part of Merck's life science business and in combination with Merck's earlier acquired Millipore Corporation, Millipore, operates as MilliporeSigma. History Sigma Chemical Company of St. Louis and Aldrich Chemical Company of Milwaukee were both American specialty chemical companies when they merged in August 1975. The company grew throughout the 1980s and 1990s, with significant expansion in fac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials are the familiar metals noticeably attracted to a magnet, a consequence of their large magnetic permeability. Magnetic permeability describes the induced magnetization of a material due to the presence of an ''external'' magnetic field, and it is this temporarily induced magnetization inside a steel plate, for instance, which accounts for its attraction to the permanent magnet. Whether or not that steel plate acquires a permanent magnetization itself, depends not only on the strength of the applied field, but on the so-called coercivity of that material, which varies greatly among ferromagnetic materials. In physics, several different types of material magnetism are distinguished. Ferromagnetism (along with the similar effect ferrimagneti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobalt(II) Squarate Dihydroxide
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal. Cobalt-based blue pigments ( cobalt blue) have been used since ancient times for jewelry and paints, and to impart a distinctive blue tint to glass, but the color was for a long time thought to be due to the known metal bismuth. Miners had long used the name ''kobold ore'' (German for ''goblin ore'') for some of the blue-pigment-producing minerals; they were so named because they were poor in known metals, and gave poisonous arsenic-containing fumes when smelted. In 1735, such ores were found to be reducible to a new metal (the first discovered since ancient times), and this was ultimately named for the ''kobold''. Today, some cobalt is produced specifically from one o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal. Cobalt-based blue pigments ( cobalt blue) have been used since ancient times for jewelry and paints, and to impart a distinctive blue tint to glass, but the color was for a long time thought to be due to the known metal bismuth. Miners had long used the name ''kobold ore'' (German for ''goblin ore'') for some of the blue-pigment-producing minerals; they were so named because they were poor in known metals, and gave poisonous arsenic-containing fumes when smelted. In 1735, such ores were found to be reducible to a new metal (the first discovered since ancient times), and this was ultimately named for the ''kobold''. Today, some cobalt is produced specifically from one of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobalt(II) Hydroxide
Cobalt(II) hydroxide or cobaltous hydroxide is the inorganic compound with the formula , consisting of divalent cobalt cations and hydroxide anions . The pure compound, often called the "beta form" (β-) is a pink solid insoluble in water. The name is also applied to a related compound, often called "alpha" or "blue" form (α-), which incorporates other anions in its molecular structure. This compound is blue and rather unstable.Xiaohe Liu, Ran Yi, Ning Zhang, Rongrong Shi, Xingguo Li, and Guanzhou Qiu (2008): "Cobalt hydroxide nanosheets and their thermal decomposition to cobalt oxide nanorings". ''Chemistry: An Asian Journal'', volume 3, issue 4, pages 732-738. Cobalt(II) hydroxide is most used as a drying agent for paints, varnishes, and inks, in the preparation of other cobalt compounds, as a catalyst and in the manufacture of battery electrodes. Preparation Cobalt(II) hydroxide precipitates as a solid when an alkali metal hydroxide is added to an aqueous solution of Co2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
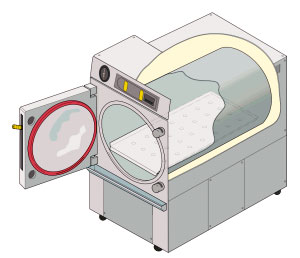
Autoclave
An autoclave is a machine used to carry out industrial and scientific processes requiring elevated temperature and pressure in relation to ambient pressure and/or temperature. Autoclaves are used before surgical procedures to perform sterilization and in the chemical industry to cure coatings and vulcanize rubber and for hydrothermal synthesis. Industrial autoclaves are used in industrial applications, especially in the manufacturing of composites. Many autoclaves are used to sterilize equipment and supplies by subjecting them to pressurized saturated steam at for around 30-60 minutes at a pressure of 15 psi (103 kPa or 1.02 atm) depending on the size of the load and the contents. The autoclave was invented by Charles Chamberland in 1879, although a precursor known as the steam digester was created by Denis Papin in 1679. The name comes from Greek ''auto-'', ultimately meaning self, and Latin ''clavis'' meaning key, thus a self-locking device. Uses Sterilization autoclav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


-chloride-hexahydrate-sample.jpg)