|
Spirotryprostatin B
Spirotryprostatin B is an indole, indolic alkaloid found in the ''Aspergillus fumigatus'' fungus that belongs to a class of naturally occurring 2,5-diketopiperazines. Spirotryprostatin B and several other indolic alkaloids (including Spirotryprostatin A, as well as other tryprostatins and cyclotryprostatins) have been found to have anti-mitosis, mitotic properties, and as such they have become of great interest as anti-cancer drugs. Because of this, the total synthesis, total syntheses of these compounds is a major pursuit of organic chemists, and a number of different syntheses have been published in the chemical literature. Total synthesis The first total synthesis was accomplished in 2000 by the Danishefsky group at Columbia University, with a number of other syntheses following shortly thereafter by Williams, Ganesan, Fuji, Carreira, Horne, Overman, and most recently Trost. From a synthetic point of view, the most challenging structural features of the molecule are the C3 spi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carreira SpirotryprostatinB Synthesis
Carreira may refer to: *Carreira (surname) *Carreira (Ribeira), a parish in the municipality of Ribeira, Galicia, Spain *Carreira (Barcelos), a parish in the municipality of Barcelos, Portugal *Carreira (Santo Tirso) Carreira is a former civil parish in the municipality of Santo Tirso, Portugal. In 2013, the parish merged into the new parish Carreira e Refojos de Riba de Ave. It is located to the south of the city of Santo Tirso in the Leça Valley of Port ..., a parish in the municipality of Santo Tirso, Portugal See also * Carreiras (Portalegre), a parish in Portalegre Municipality, Portugal {{disambiguation, geo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
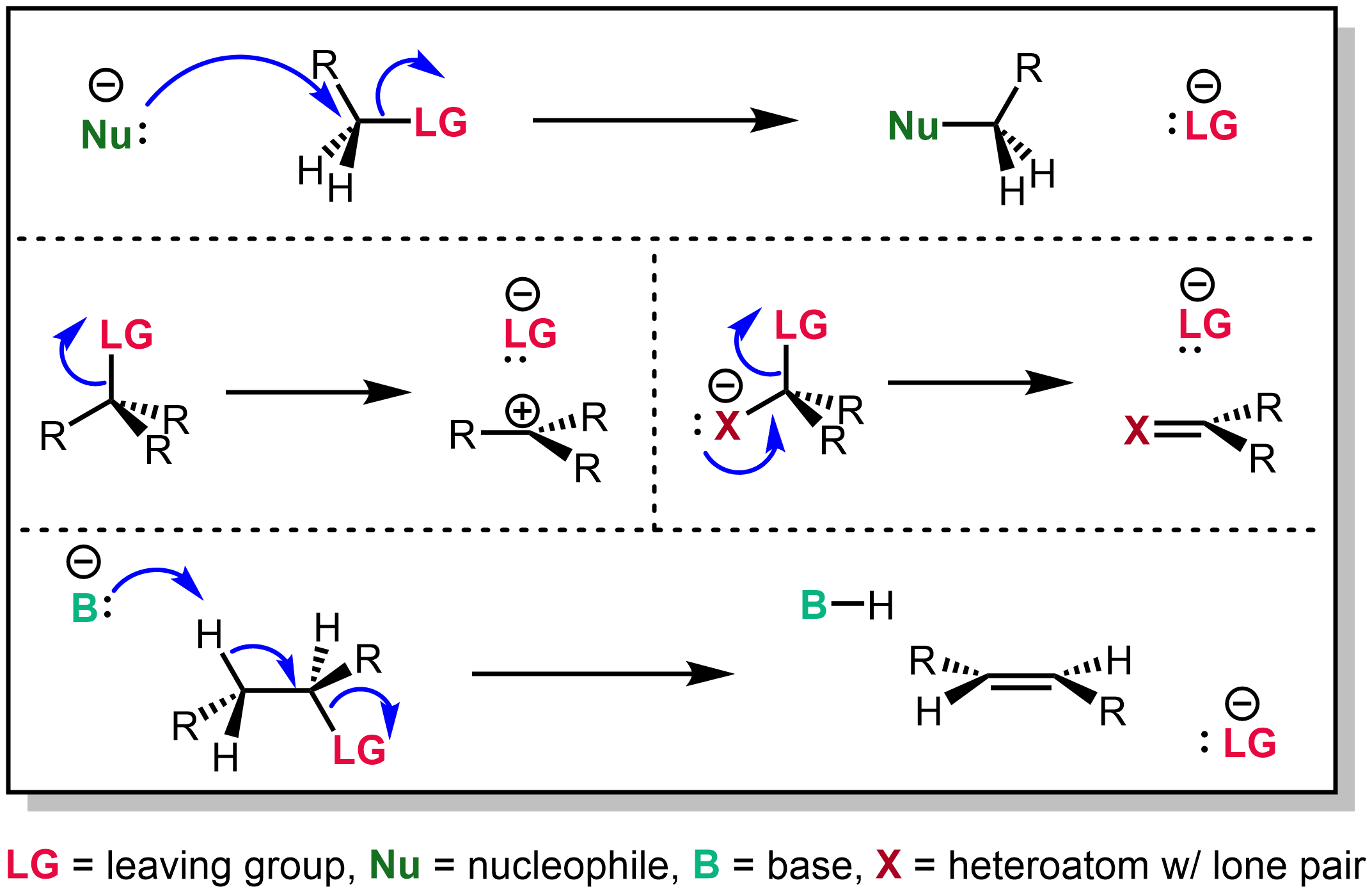
Leaving Group
In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited to a fragment that departs with a pair of electrons in heterolytic bond cleavage. In this usage, a leaving group is a less formal but more commonly used synonym of the term '' nucleofuge''. In this context, leaving groups are generally anions or neutral species, departing from a neutral or cationic substrates, respectively, though in rare cases, cations leaving from a dicationic substrate are also known. A species' ability to serve as a leaving group depends on its ability to stabilize the additional electron density that results from bond heterolysis. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters such as tosylate (TsO−), while water (H2O), alcohols (HOR), and amines (R3N) are common neutr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chirality (chemistry)
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotation (geometry), rotations, translation (geometry), translations, and some Conformational isomerism, conformational changes. This geometric property is called chirality (). The terms are derived from Ancient Greek χείρ (''cheir'') 'hand'; which is the canonical example of an object with this property. A chiral molecule or ion exists in two stereoisomers that are mirror images of each other, called enantiomers; they are often distinguished as either "right-handed" or "left-handed" by their absolute configuration or some other criterion. The two enantiomers have the same chemical properties, except when reacting with other chiral compounds. They also have the same physics, physical properties, except that they often have opposite optical activity, optical activities. A homogeneous mixture of the two enantiomers in equal parts is said to be racemic mixtu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which by definition have the same molecular formula and sequence of bonded atoms (constitution), but differ in structural formula (the three-dimensional orientations of their atoms in space). For this reason, it is also known as 3D chemistry—the prefix "stereo-" means "three-dimensionality". Stereochemistry spans the entire spectrum of organic, inorganic, biological, physical and especially supramolecular chemistry. Stereochemistry includes methods for determining and describing these relationships; the effect on the physical or biological properties these relationships impart upon the molecules in question, and the manner in which these relationships influence the reactivity of the molecules in question ( dynamic stereochemis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fuji SpirotryprostatinB Synthesis
Fuji may refer to: Places China * Fuji, Xiangcheng City (付集镇), town in Xiangcheng City, Henan Japan * Mount Fuji, the tallest mountain in Japan * Fuji River * Fuji, Saga, town in Saga Prefecture * Fuji, Shizuoka, city in Shizuoka Prefecture * Fuji Speedway, a major race track at the base of Mt Fuji People * Fuji (surname), a Japanese surname * Mr. Fuji, ring name of American professional wrestler and manager Harry Fujiwara (1934–2016) * Mr. Fuji, one of many modern monikers of the creator of Fuji musical genre, Ayinde Barrister Fictional characters * Fuji (comics), a character in the ''Stormwatch'' series Music * Mt. Fuji Jazz Festival, a jazz festival in Japan * Fuji Rock Festival, a rock festival in Japan * Fuji music, a music genre from Yorubaland of Nigeria Japanese companies * Fujifilm, a Japanese company producing cameras and photographic film * Fuji Heavy Industries, Ltd., the former name of Subaru Corporation, a Japanese company producing industrial pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diastereoselective
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter, they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two. Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another. Enantiomers of a compound with more than one stereocenter are also diastereomers of the other stereoisomers of that compound that are not their mirror image (that is, excluding the opposing enantiomer). Diastereomers h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereocenter
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups creates a new stereoisomer. Stereocenters are also referred to as stereogenic centers. A stereocenter is geometrically defined as a point (location) in a molecule; a stereocenter is usually but not always a specific atom, often carbon. Stereocenters can exist on chiral or achiral molecules; stereocenters can contain single bonds or double bonds. The number of hypothetical stereoisomers can be predicted by using 2''n'', with ''n'' being the number of tetrahedral stereocenters; however, exceptions such as meso compounds can reduce the prediction to below the expected 2''n''. Chirality centers are a type of stereocenter with four different substituent groups; chirality centers are a specific subset of stereocenters because they can only ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Bromosuccinimide
''N''-Bromosuccinimide or NBS is a chemical reagent used in radical substitution, electrophilic addition, and electrophilic substitution reactions in organic chemistry. NBS can be a convenient source of Br•, the bromine radical. Preparation NBS is commercially available. It can also be synthesized in the laboratory. To do so, sodium hydroxide and bromine are added to an ice-water solution of succinimide. The NBS product precipitates and can be collected by filtration. Crude NBS gives better yield in the Wohl-Ziegler reaction. In other cases, impure NBS (slightly yellow in color) may give unreliable results. It can be purified by recrystallization from 90 to 95 °C water (10 g of NBS for 100 mL of water). Reactions Addition to alkenes NBS will react with alkenes 1 in aqueous solvents to give bromohydrins 2. The preferred conditions are the portionwise addition of NBS to a solution of the alkene in 50% aqueous DMSO, DME, THF, or ''tert''-butanol at 0 � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomimetic Synthesis
Biomimetic synthesis is an area of organic chemical synthesis that is specifically biologically inspired. The term encompasses both the testing of a "biogenetic hypothesis" (''conjectured'' course of a biosynthesis in nature) through execution of a series of reactions designed to parallel the proposed biosynthesis, as well as programs of study where a synthetic reaction or reactions aimed at a desired synthetic goal are designed to mimic one or more ''known'' enzymic transformations of an established biosynthetic pathway. The earliest generally cited example of a biomimetic synthesis is Sir Robert Robinson's organic synthesis of the alkaloid tropinone. A more recent example is E.J. Corey's carbenium-mediated cyclization of an engineered linear polyene to provide a tetracyclic steroid ring system, which built upon studies of cationic cyclizations of linear polyenes by the Albert Eschenmoser and Gilbert Stork, and the extensive studies of the W.S. Johnson to define the requireme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ganesan SpirotryprostatinB Synthesis
Ganesan or Ganeshan ( ta, கணேசன்) is a Tamil male given name. Due to the Tamil tradition of using patronymic surnames it may also be a surname for males and females. The name is derived from the Hindu god Ganesh. Notable people Given name * Anayampatti S. Ganesan, Indian musician * C. Ganesan, Indian politician * D. Ganesan, Indian politician * Dhanpal Ganeshan (born 1994), Indian footballer * I. Ganesan, Indian politician * K. C. Ganesan, Indian politician * L. Ganesan (born 1934), Indian politician * La Ganesan, Indian politician * N. Ganesan (1932–2015), Singaporean football administrator * P. Ganesan, Indian politician * S. Ganesan, Indian politician * S. A. Ganesan, Indian politician * Saw Ganesan (1908–1982), Indian politician * Susi Ganeshan, Indian film director Surname * Ganeshan Venkataraman (born 1932), Indian physicist * Gemini Ganesan (1920–2005), Indian actor * Mano Ganesan (born 1959), Sri Lankan trade unionist and politician * Praba Gane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |