|
Sotrovimab
Sotrovimab, sold under the brand name Xevudy, is a human neutralizing monoclonal antibody with activity against severe acute respiratory syndrome coronavirus 2, known as SARS-CoV-2. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged. It was developed by GlaxoSmithKline and Vir Biotechnology, Inc. Sotrovimab is designed to attach to the spike protein of SARS-CoV-2. The most common side effects include hypersensitivity (allergic) reactions and infusion-related reactions. Although Sotrovimab was used world-wide against SARS-CoV-2, including in the United States under an FDA emergency use authorization (EUA), the FDA canceled ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SARS-CoV-2 Omicron Variant
Omicron (B.1.1.529) is a variant of SARS-CoV-2 first reported to the World Health Organization (WHO) by the Network for Genomics Surveillance in South Africa on 24 November 2021. It was first detected in Botswana and has spread to become the predominant variant in circulation around the world. Following the original BA.1 variant, several subvariants of Omicron have emerged: BA.2, BA.3, BA.4, and BA.5. Since October 2022, two subvariants of BA.5 named BQ.1 and BQ.1.1 have spread rapidly. Three doses of a COVID-19 vaccine provide protection against severe disease and hospitalisation caused by BA.1 and BA.2. The immunity effects of BA.2 are similar to those of BA.1. For three-dose vaccinated individuals, the BA.4 and BA.5 variants are more infectious than previous subvariants, making it likely, , for a new peak in COVID-19 infections to occur. __TOC__ Classification On 26 November, the WHO's Technical Advisory Group on SARS-CoV-2 Virus Evolution declared PANGO linea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coronavirus Spike Protein
Spike (S) glycoprotein (sometimes also called spike protein, formerly known as E2) is the largest of the four major structural proteins found in coronaviruses. The spike protein assembles into trimers that form large structures, called spikes or peplomers, that project from the surface of the virion. The distinctive appearance of these spikes when visualized using negative stain transmission electron microscopy, "recalling the solar corona", gives the virus family its name. The function of the spike glycoprotein is to mediate viral entry into the host cell by first interacting with molecules on the exterior cell surface and then fusing the viral and cellular membranes. Spike glycoprotein is a class I fusion protein that contains two regions, known as S1 and S2, responsible for these two functions. The S1 region contains the receptor-binding domain that binds to receptors on the cell surface. Coronaviruses use a very diverse range of receptors; SARS-CoV (which causes SARS) an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclonal Antibody
A monoclonal antibody (mAb, more rarely called moAb) is an antibody produced from a cell Lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell. Monoclonal antibodies can have monovalent affinity, binding only to the same epitope (the part of an antigen that is recognized by the antibody). In contrast, polyclonal antibodies bind to multiple epitopes and are usually made by several different antibody-secreting plasma cell lineages. Bispecific monoclonal antibodies can also be engineered, by increasing the therapeutic targets of one monoclonal antibody to two epitopes. It is possible to produce monoclonal antibodies that specifically bind to virtually any suitable substance; they can then serve to detect or purify it. This capability has become an investigative tool in biochemistry, molecular biology, and medicine. Monoclonal antibodies are being used on a clinical level for both the diagnosis and therapy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coronavirus Spike Protein
Spike (S) glycoprotein (sometimes also called spike protein, formerly known as E2) is the largest of the four major structural proteins found in coronaviruses. The spike protein assembles into trimers that form large structures, called spikes or peplomers, that project from the surface of the virion. The distinctive appearance of these spikes when visualized using negative stain transmission electron microscopy, "recalling the solar corona", gives the virus family its name. The function of the spike glycoprotein is to mediate viral entry into the host cell by first interacting with molecules on the exterior cell surface and then fusing the viral and cellular membranes. Spike glycoprotein is a class I fusion protein that contains two regions, known as S1 and S2, responsible for these two functions. The S1 region contains the receptor-binding domain that binds to receptors on the cell surface. Coronaviruses use a very diverse range of receptors; SARS-CoV (which causes SARS) an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
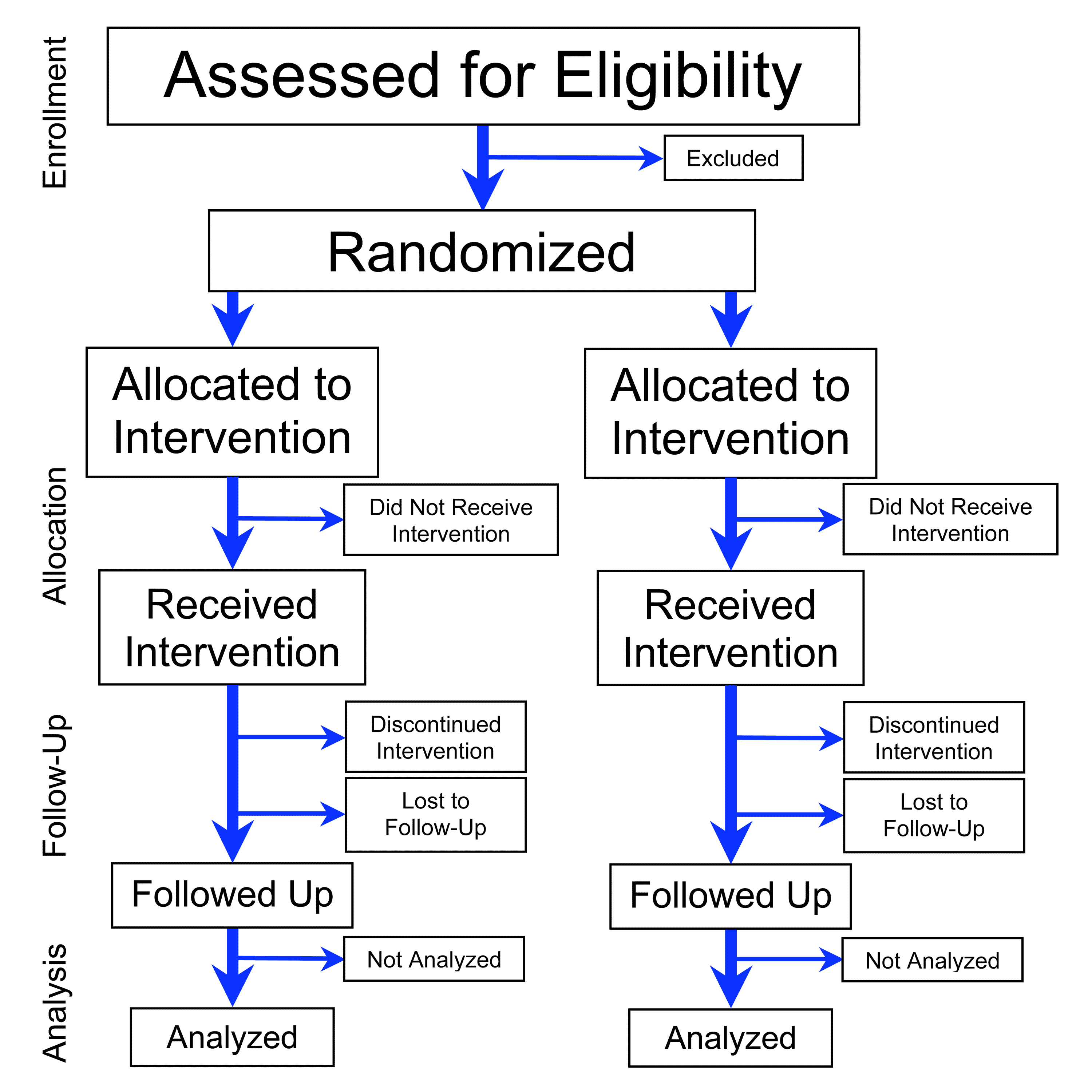
Pivotal Trial
A pivotal trial is typically a Phase III clinical trial in the multi-year process of clinical research intended to demonstrate and confirm the safety and efficacy of a treatment – such as a drug candidate, medical device or clinical diagnostic procedure – and to estimate the incidence of common adverse effects. A successful pivotal trial is required as evidence for drug marketing approval by the United States Food and Drug Administration (FDA). In drug research, a pivotal Phase III trial may be referred to as a "therapeutic confirmatory study", and is conducted in a large number (hundreds to thousands) of subjects. Such pivotal trials are also designed to discover and estimate the prevalence of common adverse events, but based on their size only have the statistical power to establish an adverse effect rate of not less than 1 in 100 subjects. In an analysis of pivotal trials on medical devices conducted between 2006 and 2013, the median duration was three years, with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Randomized Controlled Trial
A randomized controlled trial (or randomized control trial; RCT) is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures or other medical treatments. Participants who enroll in RCTs differ from one another in known and unknown ways that can influence study outcomes, and yet cannot be directly controlled. By Random assignment, randomly allocating participants among compared treatments, an RCT enables ''statistical control'' over these influences. Provided it is designed well, conducted properly, and enrolls enough participants, an RCT may achieve sufficient control over these confounding factors to deliver a useful comparison of the treatments studied. Definition and examples An RCT in clinical research typically compares a proposed new treatment against an existing Standard of care#Medical standard of care, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibody Opsonization
Antibody opsonization is a process by which a pathogen is marked for phagocytosis. Given normal inflammatory circumstances, microbial pathogen-associated molecular patterns (PAMPs) bind with the endocytic pattern recognition receptors (PRRs) of phagocytes, which mediates neutrophil mediation or macrophage phagocytosis. As well as endocytic PRRs, phagocytes furthermore express opsonin receptors such as Fc receptor and complement receptor 1 (CR1). Should the microbe be coated with opsonising antibodies or C3b complement, the co-stimulation of endocytic PRR and opsonin receptor increases the efficacy of the phagocytic process, enhancing the lysosomal elimination of the infective agent. This mechanism of antibody-mediated increase in phagocytic efficacy is named opsonization. Opsonization involves the binding of an opsonin (e.g., antibody) to an epitope on a pathogen. After opsonin binds to the membrane, phagocytes are attracted to the pathogen. The Fab portion of the antibody bin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibody-dependent Cellular Cytotoxicity
Antibody-dependent cellular cytotoxicity (ADCC), also referred to as antibody-dependent cell-mediated cytotoxicity, is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system actively lyses a target cell, whose membrane-surface antigens have been bound by specific antibodies. It is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection. ADCC is independent of the immune complement system that also lyses targets but does not require any other cell. ADCC requires an effector cell which classically is known to be natural killer (NK) cells that typically interact with immunoglobulin G (IgG) antibodies. However, macrophages, neutrophils and eosinophils can also mediate ADCC, such as eosinophils killing certain parasitic worms known as helminths via IgE antibodies. In general, ADCC has typically been described as the immune response to antibody-coated cells leading ultimatel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Efficacy
Efficacy is the ability to perform a task to a satisfactory or expected degree. The word comes from the same roots as ''effectiveness'', and it has often been used synonymously, although in pharmacology a pragmatic clinical trial#Efficacy versus effectiveness, distinction is now often made between efficacy and effectiveness. The word ''efficacy'' is used in pharmacology and medicine to refer both to the maximum response achievable from a pharmaceutical drug in research settings, and to the capacity for sufficient therapeutic effect or beneficial change in clinical settings. Pharmacology In pharmacology, efficacy () is the maximum response achievable from an applied or dosed agent, for instance, a small molecule drug. Intrinsic activity is a relative term for a drug's efficacy relative to a drug with the highest observed efficacy. It is a purely descriptive term that has little or no mechanistic interpretation. In order for a drug to have an effect, it needs to bind to its t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
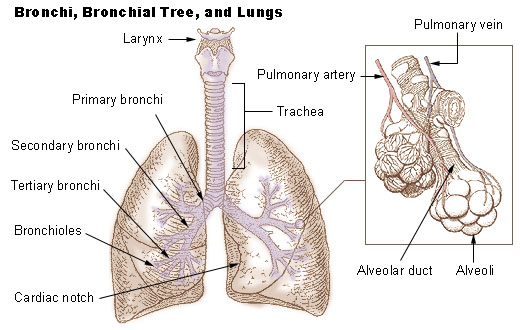
Lung
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either side of the heart. Their function in the respiratory system is to extract oxygen from the air and transfer it into the bloodstream, and to release carbon dioxide from the bloodstream into the atmosphere, in a process of gas exchange. Respiration is driven by different muscular systems in different species. Mammals, reptiles and birds use their different muscles to support and foster breathing. In earlier tetrapods, air was driven into the lungs by the pharyngeal muscles via buccal pumping, a mechanism still seen in amphibians. In humans, the main muscle of respiration that drives breathing is the diaphragm. The lungs also provide airflow that makes vocal sounds including human speech possible. Humans have two lungs, one on the left and on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interim Analysis
In clinical trials and other scientific studies, an interim analysis is an analysis of data that is conducted before data collection has been completed. Clinical trials are unusual in that enrollment of subjects is a continual process staggered in time. If a treatment can be proven to be clearly beneficial or harmful compared to the concurrent control, or to be obviously futile, based on a pre-defined analysis of an incomplete data set while the study is on-going, the investigators may stop the study early. Statistical methods The design of many clinical trials includes some strategy for early stopping if an interim analysis reveals large differences between treatment groups, or shows obvious futility such that there is no chance that continuing to the end would show a clinically meaningful effect. In addition to saving time and resources, such a design feature can reduce study participants' exposure to an inferior or useless treatment. However, when repeated significance testing o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmaceutical Distribution
The distribution of medications has special drug safety and security considerations. Some drugs require cold chain management in their distribution. The industry uses track and trace technology, though the timings for implementation and the information required vary across different countries, with varying laws and standards. Regulation Because governments regulate access to drugs, governments control drug distribution and the drug supply chain more than trade for other goods. Distribution begins with the pharmaceutical industry manufacturing drugs. From there, intermediaries in the public sector, private sector, and non-governmental organizations acquire drugs to provide them to other intermediaries. Eventually, the drugs reach different classes of consumers who use them. Good distribution practice (GDP) is a quality warranty system, which includes requirements for purchase, receiving, storage and export of drugs intended for human consumption. It regulates the division and m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |